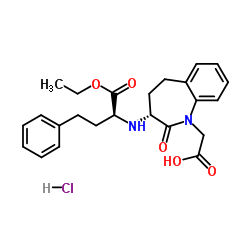
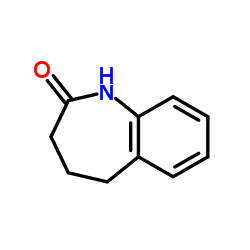
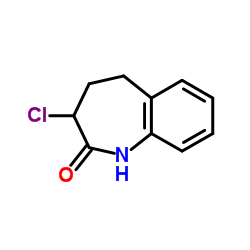
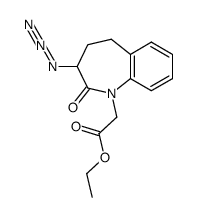
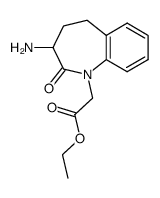
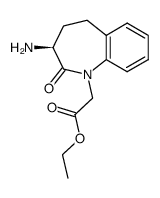
86541-78-8
| Name | benazeprilat |
|---|---|
| Synonyms |
1-carboxymethyl-3-1-carboxy-3-phenyl-(1S)-propylamino-2,3,4,5-tetrahydro-1H-(3S)-1-benzazepine-2-one
Cibacen 3-[1-carboxy-3-phenyl-(1S)-propylamino]-2,3,4,5-tetrahydro-2-oxo-1H-1-(3S)-benzazepine-1-acetic acid Benazepril Related Compound C (50 mg) ((3S)-3-[[(1S)-1- carboxy-3-phenylpropyl]amino-2,3,4,5-tetrahydro-2-oxo- 1H-1-benzazepine]-1-acetic acid) benazeprilate (3S)-3-[[(S)-1-Carboxy-3-phenylpropyl]amino]-2,3,4,5-tetrahydro-2-oxo-1H-1-benzazepine-1-acetic acid (3S)-3-[[(1S)-1-carboxy-3-phenylpropyl]aMino-2,3,4,5-tetrahydro-2-oxo-1H-1-benzazepine]-1-acetic acid 1H-1-Benzazepine-1-acetic acid,3-(1S)-1-carboxy-3-phenylpropylamino-2,3,4,5-tetrahydro-2-oxo-,(3S) Benazepril Related CoMpound C 1-carboxymethyl-3S-(1S-carboxy-3-phenylpropylamino)-2,3,4,5-tetrahydro-1H-[1]benzazepin-2-one |
| Description | Benazeprilat is an orally active and the active metabolite of benazepril, a carboxyl-containing ACE inhibitor with antihypertensive activity. Benazepril is a well-established antihypertensive agent, both in monotherapy and in combination with other classes of drugs including thiazide diuretics and calcium channel blockers. Benazepril is a first-line treatment in reducing various pathologies associated with CV risk and secondary end-organ damage[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| In Vivo | Benazeprilat (10 mg/kg, intravenous injection) and amlodipine (0.5 mg/kg, intravenous injection) in combination produce great hypotensive effect[2]. Benazepril (0.7 mg/kg, oral) markedly influences the dynamics of systemic RAAS peptides, resulting in a substantial decrease in AII and ALD while increasing PRA and AI[3]. Animal Model: Male SHR (14-16 weeks of age, 250-350 g)[2]. Dosage: 10 mg/kg Administration: I.V; once a day for 2 days. Result: Produced hypotensive effect. Animal Model: Beagle dogs (12.0-19.5 kg)[3]. Dosage: 0.7 mg/kg Administration: P.O, once a day for 5 days. Result: Effected systemic RAAS peptides. |
| References |
| Density | 1.34 g/cm3 |
|---|---|
| Boiling Point | 711.3ºC at 760 mmHg |
| Melting Point | 270-272ºC |
| Molecular Formula | C22H24N2O5 |
| Molecular Weight | 396.43600 |
| Flash Point | 384ºC |
| Exact Mass | 396.16900 |
| PSA | 106.94000 |
| LogP | 2.55050 |
| Index of Refraction | 1.643 |
| RIDADR | NONH for all modes of transport |
|---|
|
~68% 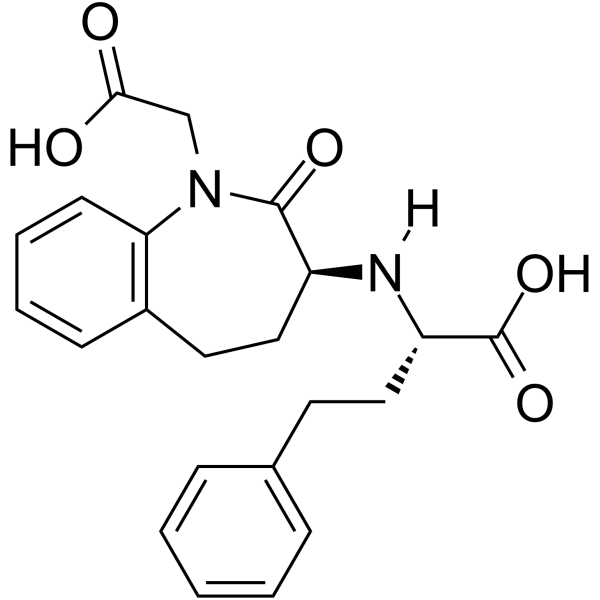
86541-78-8 |
| Literature: Watthey; Stanton; Desai; Babiarz; Finn Journal of Medicinal Chemistry, 1985 , vol. 28, # 10 p. 1511 - 1516 |
|
~% 
86541-78-8 |
| Literature: Journal of Medicinal Chemistry, , vol. 28, # 10 p. 1511 - 1516 |
|
~% 
86541-78-8 |
| Literature: Journal of Medicinal Chemistry, , vol. 28, # 10 p. 1511 - 1516 |
|
~% 
86541-78-8 |
| Literature: Journal of Medicinal Chemistry, , vol. 28, # 10 p. 1511 - 1516 |
|
~% 
86541-78-8 |
| Literature: Journal of Medicinal Chemistry, , vol. 28, # 10 p. 1511 - 1516 |
|
~% 
86541-78-8 |
| Literature: Journal of Medicinal Chemistry, , vol. 28, # 10 p. 1511 - 1516 |
|
~% 
86541-78-8 |
| Literature: Journal of Medicinal Chemistry, , vol. 28, # 10 p. 1511 - 1516 |
|
~% 
86541-78-8 |
| Literature: Journal of Medicinal Chemistry, , vol. 28, # 10 p. 1511 - 1516 |
|
~% 
86541-78-8 |
| Literature: Journal of Medicinal Chemistry, , vol. 28, # 10 p. 1511 - 1516 |
| Precursor 6 | |
|---|---|
| DownStream 0 | |