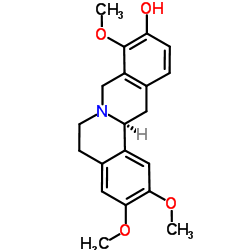
30413-84-4
| Name | (13aS)-2,3,9-Trimethoxy-5,8,13,13a-tetrahydro-6H-isoquinolino[3,2 -a]isoquinolin-10-ol |
|---|---|
| Synonyms | (13aS)-2,3,9-Trimethoxy-5,8,13,13a-tetrahydro-6H-isoquinolino[3,2-a]isoquinolin-10-ol |
| Description | Corydalmine (L-Corydalmine), an alkaloid isolated from roots of Corydalis Chaerophylla, inhibits spore germination of some plant pathogenic as well as saprophytic fungi[1]. Corydalmine acts as an oral analgesic agent, exhibiting potent analgesic activity[2]. Corydalmine alleviates Vincristine-induced neuropathic pain in mice by inhibiting an NF-κB-dependent CXCL1/CXCR2 signaling pathway[3]. |
|---|---|
| Related Catalog | |
| Target |
CXCR2 |
| In Vivo | Corydalmine (L-Corydalmine) is a potent analgesic agent, in cynomolgus monkey, beagle dog, rat and mouse liver microsomes[2]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 501.2±50.0 °C at 760 mmHg |
| Melting Point | 177-178℃ |
| Molecular Formula | C20H23NO4 |
| Molecular Weight | 341.401 |
| Flash Point | 256.9±30.1 °C |
| Exact Mass | 341.162720 |
| PSA | 51.16000 |
| LogP | 2.93 |
| Vapour Pressure | 0.0±1.3 mmHg at 25°C |
| Index of Refraction | 1.640 |
| Hazard Codes | Xi |
|---|