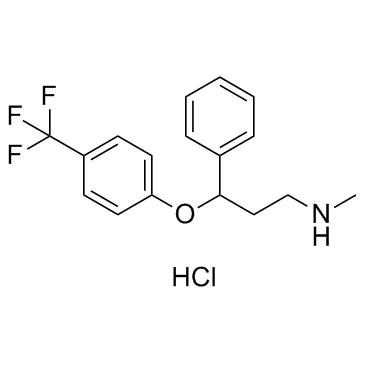
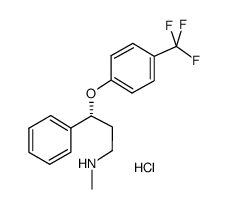
54910-89-3
| Name | Fluoxetine |
|---|---|
| Synonyms |
Adofen
MFCD00072041 AURORA KA-7692 Reneuron Fluctin Foxetin Fluoxeren Fluval fluoxetina EINECS 611-209-7 |
| Description | Fluoxetine is a selective serotonin reuptake inhibitor (SSRI) class used for antidepressant research. |
|---|---|
| Related Catalog | |
| In Vitro | Fluoxetine blocks the downregulation of cell proliferation resulting from inescapable shock (IS) of hippocampal cell[1]. Fluoxetine increases the number of newborn cells in the dentate gyrus of the hippocampus of adult rat. Fluoxetine also increases the number of proliferating cells in the prelimbic cortex[2]. Fluoxetine accelerates the maturation of immature neurons. Fluoxetine enhances neurogenesis-dependent long-term potentiation (LTP) in the dentate gyrus[3]. Fluoxetine, but not citalopram, fluvoxamine, paroxetine and sertraline, increases norepinephrine and dopamine extracellular levels in prefrontal cortex. Fluoxetine produces robust and sustained increases in extracellular concentrations of norepinephrine and dopamine after acute systemic administration[4]. |
| In Vivo | Fluoxetine treatment also reverses the deficit in escape latency observed in animals exposed to inescapable shock in adult male Sprague-Dawley rats[1]. Fluoxetine (5 mg/kg) alone increases cell proliferation in the dentate gyrus. Coadministration (fluoxetine 5 mg/kg + olanzapine) also significantly increases the number of BrdU-positive cells compared with the control group[2]. Fluoxetine combined with Olanzapine produces robust, sustained increases of extracellular levels of dopamine ([DA](ex)) and norepinephrine ([NE](ex)) up to 361% and 272% of the baseline, respectively, which are significantly greater than either drug alone[5]. |
| Animal Admin | Male Sprague-Dawley rats weighing 250-300 g are housed under a 12-hour light/12-hour dark cycle (lights on at 7:00 am, lights off at 7:00 pm) and at constant temperature (25°C) and humidity and allowed free access to food and water. For chronic drug treatments, rats are administered fluoxetine (5 mg/kg/day) or saline by intraperitoneal (IP) injection once daily and olanzapine or vehicle in the drinking water for 21 days (vehicle-treated control, fluoxetine, and olanzapine alone) plus the combination of fluoxetine and olanzapine. For combination treatment, olanzapine is chosen because fluoxetine is known to interfere with the metabolism of olanzapine and raise the blood levels by up to 4-6 times. Olanzapine is dissolved in hydrochloric acid (HCl), then adjusted back to pH 6 with 1 N sodium hydroxide to make the stock solution of 3 mg/mL concentration. The same amount of vehicle solution is added to the water for the control animals. Fluid intake is measured three times per week, and drinking bottles are replenwashed with fresh drug solution. There are no differences in fluid intake among the treatment groups. For subchronic treatment, drugs are administered exactly the same way but for a total period of 7 days. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 395.1±42.0 °C at 760 mmHg |
| Melting Point | 158ºC |
| Molecular Formula | C17H18F3NO |
| Molecular Weight | 309.326 |
| Flash Point | 192.8±27.9 °C |
| Exact Mass | 309.134064 |
| PSA | 21.26000 |
| LogP | 4.09 |
| Vapour Pressure | 0.0±0.9 mmHg at 25°C |
| Index of Refraction | 1.511 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | C,Xi |
|---|---|
| Risk Phrases | R34:Causes burns. R36/37:Irritating to eyes and respiratory system . R36/37/38:Irritating to eyes, respiratory system and skin . |
| Safety Phrases | S23-S26-S27-S36/37/39-S45-S37/39 |
| RIDADR | UN 3265 8/PG 2 |
| WGK Germany | 2 |
| Packaging Group | III |
| Hazard Class | 8 |
| HS Code | 29036990 |
| Precursor 9 | |
|---|---|
| DownStream 2 | |
| HS Code | 29036990 |
|---|

![Ethyl N-methyl-N-[3-phenyl-3-[4-(trifluoromethyl)phenoxy]propyl]carbamate structure](https://image.chemsrc.com/caspic/159/204704-95-0.png)
![methyl-[3-phenyl-3-[4-(trifluoromethyl)phenoxy]propyl]cyanamide structure](https://image.chemsrc.com/caspic/003/57226-06-9.png)