303-98-0
| Name | coenzyme Q10 |
|---|---|
| Synonyms |
coenzyme Q-10
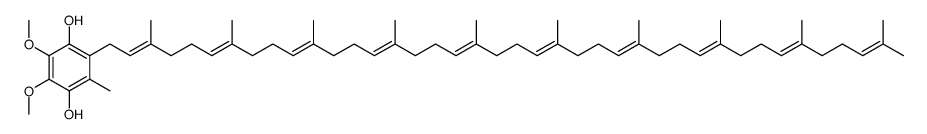
2-[(2E,6E,10E,14E,18E,22E,26E,30E,34E)-3,7,11,15,19,23,27,31,35,39-decamethyltetraconta-2,6,10,14,18,22,26,30,34,38-decaen-1-yl]-5,6-dimethoxy-3-methylcyclohexa-2,5-diene-1,4-dione CoQ10 Ube-Q Coenzyme Q Ubiquinone 50 Coenzyme Q 10 Ubiquinone 10 2-[(2E,6E,10E,14E,18E,22E,26E,30E,34E)-3,7,11,15,19,23,27,31,35,39-Decamethyl-2,6,10,14,18,22,26,30,34,38-tetracontadecaen-1-yl]-5,6-dimethoxy-3-methyl-1,4-benzoquinone Coenzyme Q10 2-[(2E,6E,10E,14E,18E,22E,26E,30E,34E)-3,7,11,15,19,23,27,31,35,39-Decamethyltetraconta-2,6,10,14,18,22,26,30,34,38-decaen-1-yl]-5,6-dimethoxy-3-methyl-1,4-benzoquinone Ensorb Liquid-Q Coenzyme Q10 Carenone ubiquinone EINECS 206-147-9 2-[(2E,6E,10E,14E,18E,22E,26E,30E,34E)-3,7,11,15,19,23,27,31,35,39-Decamethyltetraconta-2,6,10,14,18,22,26,30,34,38-decaen-1-yl]-5,6-dimethoxy-3-methylcyclohexa-2,5-dien-1,4-dion ubiquinone (50) Coenz10 neuqinon ubiquinone Q10 MFCD00042919 eiquinon Q-Gel ubidecarenone Bio-Quinon Ubiquinone-10 Coenzyme Q10,Q-10,Ubiquinone 50 Q-10 Kudesan Q-10 Ubiquinone 50 Ubiquinone-10 |
| Description | Coenzyme Q10 is an essential cofactor of the electron transport chain and a potent antioxidant agent. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| In Vitro | Coenzyme Q10 is an obligatory member of the respiratory chain in the mitochondria of all cells. Therefore, it is an essential ingredient in the formation of adenosine triphosphate (ATP), the source of energy in most cellular processes. Coenzyme Q10 is located in the mitochondria, lysosomes, and Golgi and plasma membranes, and provides an antioxidant action either by direct reaction with free radicals or by regeneration of tocopherol and ascorbate from their oxidised state[1]. Coenzyme Q10 is a popular dietary supplement because of its recognition by the public as an important nutrient in supporting human health. The rationale for the use of Coenzyme Q10 as a therapeutic agent in several cardiovascular and degenerative neurologic and neuromuscular diseases is based upon its fundamental role in mitochondrial function and cellular bioenergetics[2]. |
| In Vivo | Because of its hydrophobicity and large molecular weight, absorption of dietary Coenzyme Q10 is slow and limited. In the case of dietary supplements, solubilized Coenzyme Q10 formulations show enhanced bioavailability. The Tmax is around 6 h, with an elimination half-life of about 33 h. The reference intervals for plasma Coenzyme Q10 range from 0.40 to 1.91 mM in healthy subjects. With Coenzyme Q10 supplements there is reasonable correlation between increase in plasma Coenzyme Q10 and ingested dose up to a certain point. Animal data show that Coenzyme Q10 in large doses is taken up by all tissues including heart and brain mitochondria[2]. In 12-month-old rats administration of coenzyme Q10 results in significant increases in cerebral cortex mitochondrial concentrations of coenzyme Q10. Oral administration of coenzyme Q10 markedly attenuates striatal lesions produced by systemic administration of 3-nitropropionic acid and significantly increases life span in a transgenic mouse model of familial amyotrophic lateral sclerosis[3]. |
| Animal Admin | Rats[3] The effects of oral administration of coenzyme Q10 on 3-NP-induced striatal lesions are examined in 300- to 350-g rats (n=5-10). Animals receive either rat chow supplemented with coenzyme Q10 at 200 mg/kg or unsupplemented rat chow. After 1 week they are treated with 3-NP at a dose of 10 mgykg i.p. twice a day until either a control or treated animal became symptomatic with hindlimb dystonia, and then sacrificed in pairs[3]. Mice[3] To determine whether coenzyme Q10 exerts neuroprotective effects in a transgenic mouse model of a human neurodegenerative disorder, coenzyme Q10 (200 mg/kg) is administered orally to 16 transgenic mice overexpressing a human CuyZn superoxide dismutase (SOD1) mutation, as compared with 13 animals that receive unsupplemented rat chow. The mice used are the G1 line, which expresses high levels of human SOD with the G93A mutation. Treatment is started at 50 days after birth. Treatment is continued until mice reach end-stage disease[3]. |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 869.0±65.0 °C at 760 mmHg |
| Melting Point | 49-51 °C |
| Molecular Formula | C59H90O4 |
| Molecular Weight | 863.344 |
| Flash Point | 324.6±34.3 °C |
| Exact Mass | 862.683899 |
| PSA | 52.60000 |
| LogP | 20.93 |
| Vapour Pressure | 0.0±3.3 mmHg at 25°C |
| Index of Refraction | 1.526 |
| Storage condition | −20°C |
| Stability | Stable, but may be light or heat sensitive. Store in the dark at -20 C. Incompatible with strong oxidizing agents. |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | 22-24/25-26 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | DK3900000 |
| HS Code | 2932999099 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
| HS Code | 2932999099 |
|---|---|
| Summary | 2932999099. other heterocyclic compounds with oxygen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |