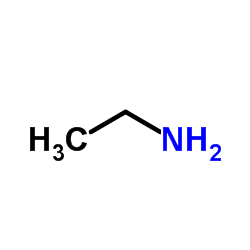
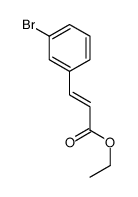
58473-74-8
| Name | trans-3-bromo-n-ethylcinnamamide |
|---|---|
| Synonyms |
MFCD00075351
cinromide trans 3-bromo-N-ethylcinnamamide Cinromide (usan/inn) m-Bromo-N-ethylcinnamamide trans-3-Brom-N-ethylzimtsaeureamid (e)-3-(3-bromophenyl)-n-ethyl-2-propenamide |
| Description | Cinromide is a broad-spectrum anticonvulsant agent. |
|---|---|
| Related Catalog | |
| In Vitro | Cinromide (10-100 μM) inhibits 5-HT-induced contractions in rat fundus strips by 46%. Cinromide (100 μM) inhibits monoamine oxidase prepared from both liver and brain of rats[1]. |
| In Vivo | Cinromide shows electroshock convulsion and leptazol(pentetrazo1)-induced convulsion in mice, with ED50s of 60 ± 11 mg/kg, 90 ± 15 mg/kg and 80 ± 15 mg/kg, 300 ± 61 mg/kg for i.p. and oral administrion, respectively. Cinromide produces a dose-related antileptazol activity with an ED50 value of 58 ± 11 mg/kg by i.p. administration in rats. Furthermore, Cinromide (75 mg/kg) significantly elevates the amount of leptazol needed to induce clonic seizures in the intravenously infused leptazol-threshold test in rats. Cinromide (300 mg/kg, i.p) shows no sifnificant effect on the anaesthetized open-chested dogs after 4 h treatment, neither in conscious dogs after 5-h oral treatment with 300 and 600 mg/kg of Cinromide[1]. Cinromide (40 mg/kg, i.v.) depresses the response of the neuron to the unconditioned maxillary nerve stimulus, increasing the latency and decreasing the number of spikes, and depresses the response of the neuron to the unconditioned maxillary nerve stimulus, increasing the latency and decreasing the number of spikes. Cinromide (20, 40, 80 mg/kg, i.v.) increases the latency of the unconditioned response and segmental inhibition dose-dependently. Cinromide decreases periventricular inhibition and EEG[2]. |
| Animal Admin | Cinromide is dissolved in propylene glycol to produce a solution containing 50 mg/mL. It is slowly injected into the femoral vein over a 3-min period. Only one neuron in each cat is studied. To evaluate the dose-response relationship, the drug is given in three cumulative doses. The interval between drug injections is 15 min. Blood samples for drug level measurement are taken 10 min after each injection. Plasma levels of cinromide and its metabolites are determined by high-performance liquid chromotography[2]. |
| References |
| Density | 1.369g/cm3 |
|---|---|
| Boiling Point | 417.5ºC at 760mmHg |
| Melting Point | 89-91ºC(lit.) |
| Molecular Formula | C11H12BrNO |
| Molecular Weight | 254.12300 |
| Flash Point | 206.3ºC |
| Exact Mass | 253.01000 |
| PSA | 29.10000 |
| LogP | 2.98930 |
| Index of Refraction | 1.591 |
| Storage condition | 2-8℃ |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| RIDADR | NONH for all modes of transport |
| RTECS | UC6314000 |
| HS Code | 2924299090 |
|
~% 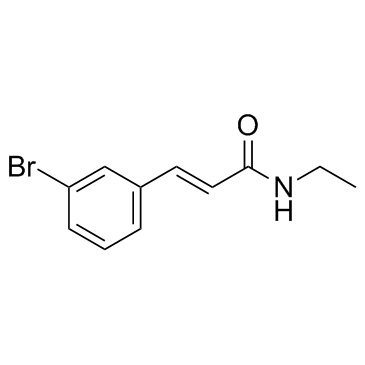
58473-74-8 |
| Literature: Burroughs Wellcome Co. Patent: US4041071 A1, 1977 ; |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
| HS Code | 2924299090 |
|---|---|
| Summary | 2924299090. other cyclic amides (including cyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |