53714-56-0
| Name | leuprolide |
|---|---|
| Synonyms |
Enantone
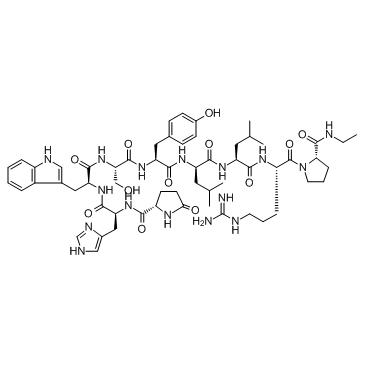
Leuprorelin Leuplin Leuprolide nghormone des-Gly10 ethylamide]gonadotropin releasing hormone MFCD00167544 LH-RH LEUPROLIDE Carcinil 5-Oxo-L-prolyl-L-histidyl-L-tryptophyl-L-seryl-L-tyrosyl-D-leucyl-L-leucyl-L-arginyl-N-ethyl-L-prolinamide pGlu-His-Trp-Ser-Tyr-D-Leu-Leu-Arg-Pro-NHC2H5 Lucrin <D-Leu6,DesGly10> LHRH ethylamide LEUPROPELIN Leuprorelin Acetate GLP-HIS-TRP-SER-TYR-DLEU-LEU-ARG-PRO-NHET: GLP-HWSY-DL-LRP-NHET |
| Description | Leuprolide is an agonist at pituitary GnRH receptors. Target: GnRH receptorLeuprorelin is a gonadotrophin-releasing hormone (GnRH) analogue used to treat a wide range of sex hormone-related disorders including advanced prostatic cancer, endometriosis and precocious puberty. Leuprorelin acts primarily on the anterior pituitary, inducing a transient early rise in gonadotrophin release. With continued use, Leuprorelin causes pituitary desensitisation and/or down-regulation, leading to suppressed circulating levels of gonadotrophins and sex hormones. [1] By interrupting the normal pulsatile stimulation of, and thus desensitizing, the GnRH receptors, it indirectly downregulates the secretion of gonadotropins luteinizing hormone (LH) and follicle-stimulating hormone (FSH), leading to hypogonadism and thus a dramatic reduction in estradiol and testosterone levels in both sexes. [2] |
|---|---|
| Related Catalog | |
| References |
[2]. Leuprorelin |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Molecular Formula | C59H84N16O12 |
| Molecular Weight | 1209.40 |
| PSA | 466.34000 |
| LogP | 0.41 |
| Index of Refraction | 1.682 |
| Storage condition | −20°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H360 |
| Precautionary Statements | P201-P280-P308 + P313 |
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | OH6390000 |
|
~% 
53714-56-0 |
| Literature: US2006/276626 A1, ; Page/Page column 13 ; |
