121524-09-2
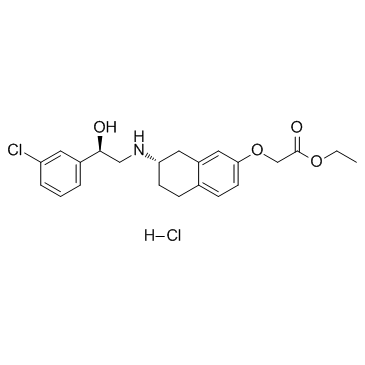
| Name | ethyl 2-[[(7S)-7-[[(2R)-2-(3-chlorophenyl)-2-hydroxyethyl]amino]-5,6,7,8-tetrahydronaphthalen-2-yl]oxy]acetate,hydrochloride |
|---|---|
| Synonyms |
C22H26ClNO4.HCl
Amibegron hydrochloride N-(7-Hydroxy-1,2,3,4-tetrahydronaphth-2-yl)-2-hydroxy-2-(3-chlorophenyl)ethanol Amibegron HCl Acetic acid,((7-((2-(3-chlorophenyl)-2-hydroxyethyl)amino)-5,6,7,8-tetrahydro-2-naphthalenyl)oxy)-,ethyl ester,hydrochloride,(R-(R*,S*)) |
| Description | Amibegron hydrochloride is a selective β3-adrenoceptor agonist, with an EC50 of 3.5 nM for β-adrenoceptor in rat colon; Amibegron hydrochloride has anxiolytic and antidepressant activity. |
|---|---|
| Related Catalog | |
| Target |
EC50: 3.5 nM (β-adrenoceptor, from rat colon), 499 nM (β-adrenoceptor, from rat uterus)[1], 1.2 μM (β2-adrenoceptor, from cerebellum), 4.6 μM (β1-adrenoceptor1, from cortex)[2] |
| In Vitro | Amibegron hydrochloride (SR 58611A) is a selective β-adrenoceptor agonist, with an EC50 of 3.5 nM for β-adrenoceptor in rat colon, and 499 nM in rat uterus[1]. Amibegron hydrochloride (SR 58611A) shows little effect on β1- and β2-adrenoceptors, 5-HT uptake, noradrenaline (NA) uptake, and dopamine (DA) uptake from rat brain tissue, with IC50s of 4.6 and 1.2, 0.58, 2.5 and 3.2 μM, respectively; exhibits no effect on 5-HT1A, 5-HT2, MAO-A and MAO-B (IC50 > 10 μM)[2]. |
| In Vivo | Amibegron hydrochloride (SR 58611A, 0.1 to 0.3 mg/kg, i.p.) potentiates the toxicity produced by yohimbine in mice. Amibegron hydrochloride (0.6 and 2 mg/kg, i.p.) is also active in the learned helplessness model of antidepressant-like activity in rats. However, Amibegron hydrochloride exhibits no effect on the spontaneous locomotor activity of mice at up to 10 mg/kg and of rats at up tp 30 mg/kg[2]. Amibegron hydrochloride (3 and 10 mg/kg, p.o.) increases the synthesis of 5-HT and tryptophan (Trp) levels in several rodent brain areas such as cortex, hippocampus, hypothalamus, striatum. In addition, Amibegron hydrochloride (10 mg/kg, p.o.) promotes the release of 5-HT in rat prefrontal cortex. Systemic (3 mg/kg, i.v.) or chronic administration of SR58611A (10 mg/kg, p.o.) does not affect the activity of serotonergic neurons in the rat dorsal raphe nucleus[3]. |
| Animal Admin | Mice[2] This test is performed on groups of 10-20 mice. Amibegron hydrochloride (0.1 to 0.3 mg/kg) or vehicle are administered i.p. 30 min before the administration of yohimbine. Yohimbine hydrochloride is administered s.c. at a dose of 30 mg/kg always at the same time of day, between 1.30 and 3.30 p.m. Lethality is recorded the next morning at 9 a.m[2]. Rats[2] The rats (n = 10 per group) are treated randomly according to one of the following protocols: the control sample, which receives no shock, is given vehicle; experimental animals with inescapable shock are treated daily with vehicle or Amibegron hydrochloride (up to 30 mg/kg). Animals are treated orally over 5 consecutive days, i.e. 6 h after shock pretreatment on day 1, and then twice per day, a half dose in the morning (30 min before shuttle-box session) and a half dose in the afternoon (except on the 5th day). Statistical analysis is performed on themean number of escape failures using a two-way analysis of variance followed by Dunnett's test[2]. |
| References |
| Boiling Point | 576ºC at 760mmHg |
|---|---|
| Molecular Formula | C22H27Cl2NO4 |
| Molecular Weight | 440.36000 |
| Flash Point | 302.2ºC |
| Exact Mass | 439.13200 |
| PSA | 67.79000 |
| LogP | 4.65530 |
| Vapour Pressure | 4.14E-14mmHg at 25°C |
| Symbol |

GHS09 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H410 |
| Precautionary Statements | P273-P501 |
| RIDADR | UN 3077 9 / PGIII |