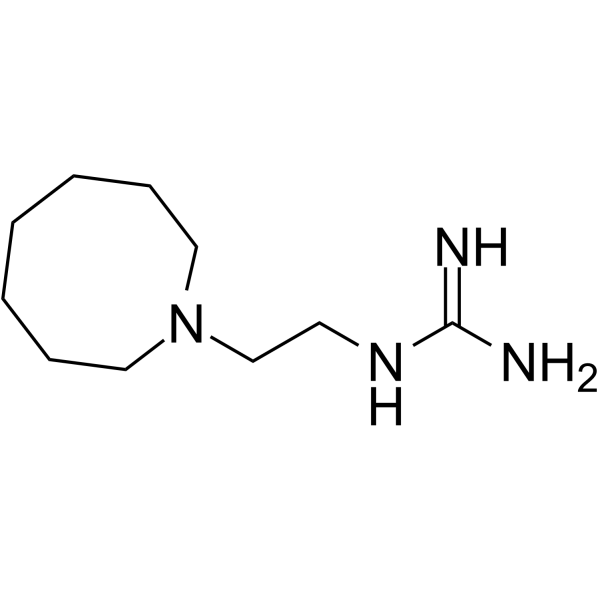
55-65-2
| Name | guanethidine |
|---|---|
| Synonyms |
Guanethidinum
Octadine GUANETHIDINE (2-<Octahydroazocinyl-(1)>-ethyl)-guanidin Oktadin [2-(hexahydro-1-(2H)-azocinyl)ethyl]guanidine Isobarin Sanotensin 2-[2-(azocan-1-yl)ethyl]guanidine Oktatenzin (2-azocan-1-yl-ethyl)-guanidine Ismelin Eutensol guanethedine Abapresin |
| Description | Guanethidine sulphate was synthesized in 1959. Guanethidine is thought to lowing blood pressure by interfering with the metabolism of chemical transmitter substances in post-ganglionic sympathetic nerve fibres. |
|---|---|
| In Vitro | Ablation of sympathetic fibers is associated with a loss of rat endothelial cell marker (RECA), but no significant effect of guanethidine was found on the survival of endothelial cells and mesenchymal stem cells in vitro[1]. . |
| In Vivo | Guanethidine (30 mg/kg, s.c., 1 h) unaffected IL-18 hypernociception in TNFR1(-/-) mice as a sympathetic blocker[2]. . Animal Model: Wild-type (WT) Balb/c,TNFR1(-/-)and IFN-γ-γ(-/-) mice[2]. Dosage: 30 mg/kg Administration: Guanethidine (30 mg/kg, s.c., 1 h, diluted in saline) Result: Pre-treatment with guanethidine (sympathetic blocker) unaffected IL-18 hypernociception in TNFR1(-/-) mice. |
| References |
| Density | 1.13g/cm3 |
|---|---|
| Boiling Point | 345.6ºC at 760mmHg |
| Molecular Formula | C10H22N4 |
| Molecular Weight | 198.30800 |
| Flash Point | 162.8ºC |
| Exact Mass | 198.18400 |
| PSA | 65.14000 |
| LogP | 1.86440 |
| Index of Refraction | 1.4910 (estimate) |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|