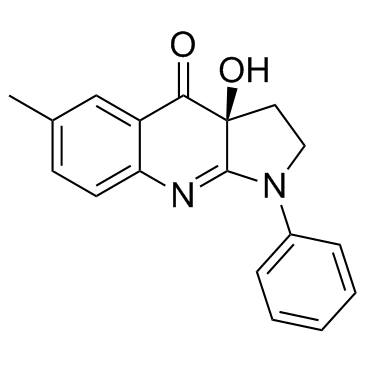
856925-71-8
| Name | (S)-(-)-Blebbistatin |
|---|---|
| Synonyms |
(3aS)-3a-Hydroxy-6-methyl-1-phenyl-1,2,3,3a,4a,8a-hexahydro-4H-pyrrolo[2,3-b]quinolin-4-one
(3aS)-3a-hydroxy-6-methyl-1-phenyl-2,3-dihydropyrrolo[2,3-b]quinolin-4-one |
| Description | (-)-Blebbistatin is an S enantiomer of blebbistatin. Blebbistatin is a potent and selective myosin II inhibitor with IC50s ranging from 0.5 to 5 μM. |
|---|---|
| Related Catalog | |
| Target |
IC50: 0.5 to 5 μM (myosin II)[1] |
| In Vitro | Blebbistatin potently inhibits several striated muscle myosins as well as vertebrate nonmuscle myosin IIA and IIB with IC50 values ranging from 0.5 to 5 μM. Smooth muscle myosin is only poorly inhibited (IC50=80 μM)[1]. Blebbistatin does not compete with nucleotide binding to the skeletal muscle myosin subfragment-1. The inhibitor preferentially binds to the ATPase intermediate with ADP and phosphate bound at the active site, and it slows down phosphate release. It blocks the myosin heads in a products complex with low actin affinity[2]. In culture-activated hepatic stellate cells, blebbistatin is found to change both cell morphology and function. Stellate cells become smaller, acquire a dendritic morphology and have less myosin IIA-containing stress fibres and vinculin-containing focal adhesions. Blebbistatin impairs silicone wrinkle formation, reduces collagen gel contraction and blocks endothelin-1-induced intracellular Ca2+ release. It promotes wound-induced cell migration[3]. |
| In Vivo | Blebbistatin dose-dependently and completely relax both KCl- and carbachol-induced rat detrusor and endothelin-1-induced human bladder contraction. Pre-incubation with 10 μM blebbistatin attenuates carbachol responsiveness by 65% while blocking electrical field stimulation-induced bladder contraction reaching 50% inhibition at 32 Hz[4]. |
| Cell Assay | Freshly isolated HSCs are replated on 96-well plate. At day 3, medium is replaced by serum-free medium and cells are starved overnight, treated with or without blebbistatin (25 μM) for 2 h followed by stimulation with platelet-derived growth factor-BB (20 ng/mL). After an overnight incubation, the WST-1 cell proliferation assay are performed[3]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 486.7±55.0 °C at 760 mmHg |
| Melting Point | 210-212ºC |
| Molecular Formula | C18H16N2O2 |
| Molecular Weight | 292.33 |
| Flash Point | 248.1±31.5 °C |
| PSA | 52.90000 |
| LogP | 0.93 |
| Vapour Pressure | 0.0±1.3 mmHg at 25°C |
| Index of Refraction | 1.681 |
| Storage condition | Desiccate at +4°C |
| Water Solubility | DMSO: soluble5mg/mL |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H312-H315-H317-H319-H332-H335 |
| Precautionary Statements | P261-P280-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xn |
| Risk Phrases | 20/21/22-36/37/38 |
| Safety Phrases | 26-36/37 |
| RIDADR | NONH for all modes of transport |