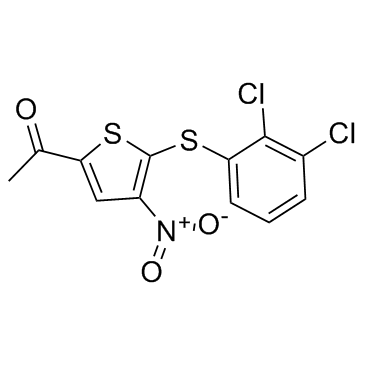
882257-11-6
| Name | 1-[5-(2,3-dichlorophenyl)sulfanyl-4-nitrothiophen-2-yl]ethanone |
|---|---|
| Synonyms |
1-{5-[(2,3-Dichlorophenyl)sulfanyl]-4-nitro-2-thienyl}ethanone
QC-8200 1-{5-[(2,3-dichlorophenyl)thio]-4-nitro-2-thienyl}ethan-1-one 1-[5-(2,3-dichlorophenylsulfanyl)-4-nitro-2-thienyl]ethanone 1-(5-(2,3-dichlorophenylthio)-4-nitrothiophen-2-yl)ethanone P005091 |
| Description | P005091 is a selective and potent inhibitor of ubiquitin-specific protease 7 (USP7) with an EC50 of 4.2 μM. |
|---|---|
| Related Catalog | |
| Target |
EC50: 4.2 μM (USP7) |
| In Vitro | P005091 is a trisubstituted thiophene with dichlorophenylthio, nitro, and acetyl substituents mediating anti-USP7 activity. P005091 exhibits potent, specific, and selective deubiquitylating activity against USP7. In contrast, P005091 does not inhibit other DUBs or other families of cysteine proteases tested (EC50 > 100 mM). P005091 inhibits the labeling of USP7 with HA-UbVME in a concentration-dependent manner. USP7-mediated cleavage of high molecular weight polyubiquitin chains is inhibited in a dose-dependent manner by P005091. Moreover, P005091 inhibits USP7- but not USP2- or USP8-mediated cleavage of poly K48-linked ubiquitin chains. USP7 inhibition by P005091 induces HDM2 polyubiquitylation and accelerates degradation of HDM2. P005091 inhibits USP7 deubiquitylating activity, without blocking proteasome activity in MM Cells. P005091 inhibits growth in MM cells and overcomes bortezomib-resistance. P005091 induces a dose-dependent decrease in viability of various MM cell lines, including those that are resistant to conventional therapies dexamethasone (Dex) (MM.1R), doxorubicin (Dox-40), or melphalan (LR5) (IC50 range 6-14 μM). P005091 overcomes bone marrow stromal cell-induced growth of MM Cells. P005091 decreases HDM2 and HDMX, as well as upregulated p53 and p21 levels. Overall, P005091-induced cytotoxicity is mediated in part via HDM2-p21 signaling axis and although p53 is upregulated in response to P005091 treatment, the cytotoxic activity of P005091 is not dependent on p53[1]. |
| In Vivo | In animal tumor model studies, P005091 is well tolerated, inhibits tumor growth, and prolongs survival. Combining P005091 with lenalidomide, HDAC inhibitor SAHA, or dexamethasone triggers synergistic anti-MM activity[1]. |
| Kinase Assay | Recombinant enzymes in 20 mM Tris-HCl (pH 8.0), 2 mM CaCl2, and 2 mM β-mercaptoethanol are incubated with dose ranges of P005091 for 30 min in a 96-well plate before the addition of Ub-PLA2 and NBD C6-HPC or Ub-EKL and EKL substrate. The liberation of a fluorescent product within the linear range of the assay is monitored using a Perkin Elmer Envision fluorescence plate reader. Vehicle (2% [v/v] DMSO) and 10 mM N-ethylmaleimide (NEM) are included as controls. |
| Animal Admin | For the animal study, P005091 is dissolved in 4% NMP(N-methyl-2-Pyrrolidone), 4% Tween-80, and 92% Milli-Q water at a final concentration of 2 mg/mL. The human plasmacytoma xenograft model is performed as previously described. CB-17 SCID-mice are subcutaneously inoculated with MM.1S, ARP-1, or RPMI-8226 cells in 100 μL of serum free RPMI-1640 medium. When tumors are measurable (100-180 mm3), mice are randomized into treatment groups. In the SCID-hu model, human fetal bone grafts are subcutaneously implanted into SCID mice. Four weeks after bone implantation, INA-6 cells are injected directly into the fetal bone implant in SCID mice; and as a measure of tumor burden, mouse sera samples are analyzed for shIL-6R by ELISA. Upon detection of shIL-6R, mice are treated with vehicle or P005091, and mouse serum is analyzed for alterations in shIL-6R levels. |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 452.4±45.0 °C at 760 mmHg |
| Molecular Formula | C12H7Cl2NO3S2 |
| Molecular Weight | 348.225 |
| Flash Point | 227.4±28.7 °C |
| Exact Mass | 346.924438 |
| PSA | 116.43000 |
| LogP | 5.02 |
| Appearance | light yellow to dark yellow |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.685 |
| Storage condition | 2-8°C |
| Water Solubility | DMSO: soluble10mg/mL, clear |
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301-H413 |
| Precautionary Statements | P301 + P310 |
| Hazard Codes | T |
| Risk Phrases | 25 |
| Safety Phrases | 45 |
| RIDADR | UN 2811 6.1 / PGIII |
| Precursor 3 | |
|---|---|
| DownStream 0 | |