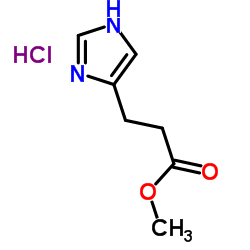
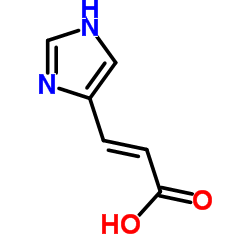
3465-72-3
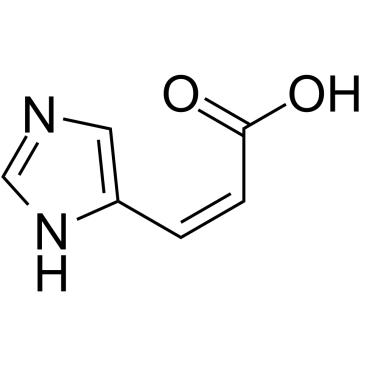
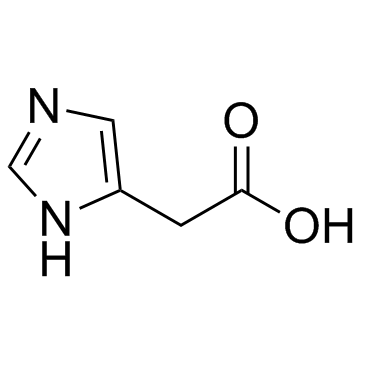
| Name | (2E)-3-(1H-Imidazol-4-yl)acrylic acid |
|---|---|
| Synonyms |
Trans-Urocanic acid
(E)-3-(1H-Imidazol-5-yl)-2-propenoic acid (E)-urocanic acid E-3-(imidazol-4-yl)propenoic acid (2E)-3-(1H-Imidazol-4-yl)acrylic acid (Z)-4-pyridalacetophenone trans-4-imidazoleacrylic acid (2E)-3-(1H-imidazol-4-yl)prop-2-enoic acid (E)-3-(1H-imidazol-4-yl)acrylic acid 1-phenyl-3-(4-pyridinyl)-2-propen-1-one Urocanic acid 3-(1H-imidazol-4-yl)prop-2-enoic acid (E)-3-(1H-imidazol-4-yl)-2-propenoic acid Urocanate (E)-3-(imidazol-4-yl)acrylic acid E-3-(4-Pyridyl)-1-phenylprop-2-en-1-on 4-Imidazoleacrylic Acid (2E)-3-(1H-Imidazol-5-yl)acrylic acid |
| Description | trans-urocanic acid (trans-UCA), a natural epidermal constituent, inhibits human natural killer cell (NK) activity in vitro. trans-urocanic acid is active in regulating an immune function[1]. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| In Vitro | trans-urocanic acid (trans-UCA) partially inhibits cytotoxic function of IL-2-activated NK cells and reduces IL-2-induced activation of NK cells[1]. trans-urocanic acid (trans-UCA;100 μg/mL) slightly decreases cell proliferation and viability of primary human keratinocytes[2]. Cell Proliferation Assay[2] Cell Line: Primary human keratinocytes Concentration: 100 μg/mL Incubation Time: 24 hours Result: Decreased cell proliferation and viability. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 456.9±20.0 °C at 760 mmHg |
| Molecular Formula | C6H6N2O2 |
| Molecular Weight | 138.124 |
| Flash Point | 230.1±21.8 °C |
| Exact Mass | 138.042923 |
| PSA | 65.98000 |
| LogP | 0.01 |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.674 |
| Storage condition | 2-8C |
| HS Code | 2933290090 |
|---|
|
~% 
3465-72-3 |
| Literature: Masuda Yakugaku Zasshi, 1956 , vol. 76, p. 325,327, 328 Chem.Abstr., 1956 , p. 13880 |
|
~% 
3465-72-3 |
| Literature: Langer, Martin; Pauling, Andrea; Retey, Janos Angewandte Chemie, 1995 , vol. 107, # 13/14 p. 1585 - 1587 |
|
~% 
3465-72-3 |
| Literature: Edlbacher; v.Bidder Hoppe-Seyler's Zeitschrift fuer Physiologische Chemie, 1942 , vol. 276, p. 126,128 Full Text Show Details Masuda Yakugaku Zasshi, 1956 , vol. 76, p. 325,327, 328 Chem.Abstr., 1956 , p. 13880 |
|
~% 
3465-72-3 |
| Literature: Valeev; Salikhov; Krasnoslobodtseva; Sharipov; Spirikhin; Tolstikov Chemistry of Natural Compounds, 2007 , vol. 43, # 2 p. 143 - 148 |
|
~% 
3465-72-3 |
| Literature: Horii et al. Yakugaku Zasshi, 1954 , vol. 74, p. 408 Chem.Abstr., 1955 , p. 5451 |
|
~% 
3465-72-3 |
| Literature: Kpissay, Armah; Kuhl, C. Nicole; Mohammad, Taj; Haber, Ken; Morrison, Harry Tetrahedron Letters, 1997 , vol. 38, # 49 p. 8435 - 8438 |
| Precursor 6 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933290090 |
|---|---|
| Summary | 2933290090. other compounds containing an unfused imidazole ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |