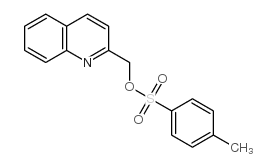
120128-20-3
| Name | 2-[[4-[[2-(2H-tetrazol-5-ylmethyl)phenyl]methoxy]phenoxy]methyl]quinoline |
|---|---|
| Synonyms |
c25h21n5o2
RG-12525 |
| Description | RG-12525 is a a specific, competitive and orally effective antagonist of the peptidoleukotrienes, LTC4, LTD4 and LTE4, inhibiting LTC4-, LTD4- and LTE4-inducd guinea pig parenchymal strips contractions, with IC50s of 2.6 nM, 2.5 nM and 7 nM, respectively; RG-12525 is also a peroxisome proliferator-activated receptor gamma (PPAR-gamma) agonist with IC50 of appr 60 nM and a potent inhibitor of CYP3A4, with a Ki value of 0.5 µM. |
|---|---|
| Related Catalog | |
| Target |
LTD4:2.5 nM (IC50, Guinea pig) LTC4:2.6 nM (IC50, Guinea pig) LTE4:7 nM (IC50, Guinea pig) PPARγ:60 nM (IC50) CYP3A4:0.5 μM (Ki) |
| In Vitro | RG 12525 competitively inhibits 3H-LTD4 binding to lung membranes (Ki = 3.0 +/- 0.3 nM) and competitively antagonizes the spasmogenic activity of LTC4, LTD4 and LTE4 on lung strips (KB values = 3 nM) with greater than 8000 fold selectivity[1]. RG 12525 (2.5 µM or 25 µM) inhibits the microsomal activity of CYP2C9 and -3A4, but does not significantly inhibit CYP1A2,-2A6, -2C19, or -2D6. RG 12525 (25 µM) also causes a substantial amount of inhibition at the 5 and 10 µM midazolam concentrations[2]. |
| In Vivo | RG 12525 orally inhibits LTD4 induced wheal formation (ED50 = 5 mg/kg with a t1/2 = 10 hrs at 9 mg/kg), LTD4 induces bronchoconstriction (ED50 = 0.6 mg/kg), and anaphylactic death (ED50 = 2.2 mg/kg with a t1/2 = 7 hrs at 10 mg/kg) and antigen induces bronchoconstriction (ED50 = 0.6 mg/kg)[1]. RG 12525 inhibits antigen-induced mortality in the systemic anaphylaxis model with an ED50 (95% confidence interval) = 2.2 (0.8-6.4) mg/kg. RG 12525 also protects against LTD4-induced bronchoconstriction in a model measuring changes in pulmonary function with an ED50 = 0.6 (0.4-1.0) mg/kg[3]. |
| References |
| Density | 1.308g/cm3 |
|---|---|
| Boiling Point | 667ºC at 760 mmHg |
| Molecular Formula | C25H21N5O2 |
| Molecular Weight | 423.46700 |
| Flash Point | 227.3ºC |
| Exact Mass | 423.17000 |
| PSA | 85.81000 |
| LogP | 4.49670 |
| Vapour Pressure | 1.17E-17mmHg at 25°C |
| Index of Refraction | 1.681 |
| Storage condition | 2-8℃ |
| Precursor 6 | |
|---|---|
| DownStream 0 | |
![[2-(Hydroxymethyl)phenyl]acetonitrile structure](https://image.chemsrc.com/caspic/214/67519-22-6.png)