182410-00-0
| Name | Captisol |
|---|---|
| Synonyms |
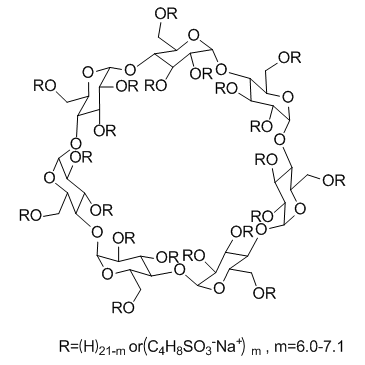
Disodium 4-({36,38,39,40,41,42,43,44,45,46,47,48,49-tridecahydroxy-5,10,15,20,25,30-hexakis(hydroxymethyl)-35-[(4-sulfonatobutoxy)methyl]-2,4,7,9,12,14,17,19,22,24,27,29,32,34-tetradecaoxaoctacyclo[31 ;.2.2.2.2.2.2.2.2]nonatetracont-37-yl}oxy)-1-butanesulfonate
CS-0731 Sodium Sulphobutylether-beta-cyclodextrin SBE-Beta-CD sulfobutyl ether-beta-cyclodextrin sodium salt SBE-β-CD |
| Description | SBE-β-CD is a sulfobutylether β-cyclodextrin derivative used as an excipient or a formulating agent to increase the solubility of poorly soluble drugs. |
|---|---|
| Related Catalog | |
| In Vitro | SBE-β-CD is a chemically modified β-CD that is a cyclic hydrophilic oligosaccharide which is negatively charged in aqueous media. β-CD functioned is a solubilizer only at low concentrations, whereas SBE7-β-CD exhibits strong solubilizing effects over a wide concentration range[1]. |
| In Vivo | SBE-β-CD is a derivatized form of β-cyclodextrin that has been developed as a safe and effective solubilizing agent for drugs being administered by parenteral and other routes (including oral). SBE-β-CD is a cyclic carbohydrate comprised of seven glucose molecules; the resulting truncated cone-like structure being further derivatized with an average of seven sulfobutyl ether groups[2]. The calorimetric data for the Compound 1/SBE-β-CD complex indicates an extremely strong interaction, with an association constant of 2.3±(0.2)×106M-1 at 25°C and 1.6±(0.2)×106M-1 at 37°C[3]. SBE-β-CD alone evokes a mild cardio-depressant effect independent of cocaine treatment (p=0.0001 compared to baseline) but attenuates further cocaine-induced decreases in RPP, dP/dtmax, and dP/dtmaxabs at high cocaine concentrations. No significant effect is seen on line pressure SBE-β-CD alleviates the most pronounced cardiac depression for RPP, dP/dtmax, and dP/dtmaxabs. This differential effect of SBE-β-CD at low and high concentrations produces an interaction effect in the two-way ANOVA for RPP (p<0.0001), dP/dtmax (p=0.0001), and dP/dtmaxabs (p=0.0015), and prevents any overall treatment effect. Infusing SBE-β-CD also attenuates the cardiac depression associated with cocaethylene toxicity for RPP and dP/dtmax. No differences are observed between ethanol-treated controls and cocaethylene plus SBE-β-CD groups[4]. |
| Animal Admin | Rats[3] A 300 g rat is administered with 1 mL of a 0.1 M SBE-β-CD solution containing 5.64 mg of Compound 1, and assuming an extracellular volume of 90 mL, less than 0.1% of the complex would rapidly dissociate due to the initial effects of dilution. This calculation, combined with the changing blood to plasma ratio in the presence of SBE-β-CD, provides a reasonable explanation for the observed differences in the blood and plasma profiles of Compound 1 after intravenous administration in either the cyclodextrin or cyclodextrin-free formulations. After IV administration of the cyclodextrin formulation, Compound 1 would initially be prevented from distributing into erythrocytes thereby resulting in a whole blood to plasma ratio of less than one. Subsequently, clearance of SBE-β-CD from the circulation would lead to changes in the complexation equilibrium such that the unbound fraction of Compound 1 would increase, thereby reestablishing normal blood to plasma partitioning (i.e. in favour of whole blood) and clearance. |
| References |
| Molecular Formula | C50H84Na2O41S2 |
|---|---|
| Molecular Weight | 1451.287 |
| Exact Mass | 1450.372437 |
| PSA | 663.21000 |
| Appearance | Solid powder | White to off white |
| Storage condition | 2-8℃ |
| Water Solubility | Soluble in water.Insoluble in acetone, methanol, chloroform. |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H317 |
| Precautionary Statements | P280 |
| RIDADR | NONH for all modes of transport |
| RTECS | GU2293470 |