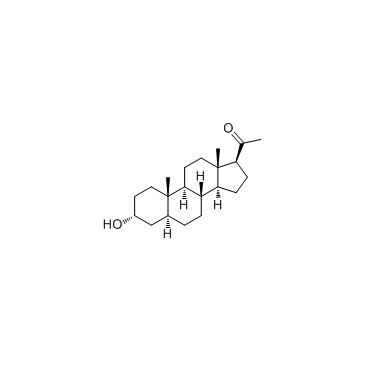
516-54-1
| Name | 3α-hydroxy-5α-pregnan-20-one |
|---|---|
| Synonyms |
pregnanalone
PREGNAN-3A-OL-20-ONE (+)-3α-Hydroxy-5α-pregnan-20-one (3α)-3-Hydroxypregnan-20-one Butabindide oxalate 3α,5α-THP (3α)-Allopregnanolone 5-Pregnan-3-ol-20-one 3alpha-hydroxy-5alpha-pregnan-20-one MFCD00003677 UNII:S39XZ5QV8Y Pregnanolone II 3alpha-hydroxy-5beta-pregnan-20-one ALLOPREGNAN-3ALPHA-OL-20-ONE Brexanolone Allopregnanolone 3α-Hydroxy-5α-pregnan-20-one 3a-Hydroxy-5a-pregnane-20-one (3α,5α)-3-Hydroxypregnan-20-one 3a-Hydroxy-5a-pregnan-20-one (+)-3a-Hydroxy-5a-pregnan-20-one 3α,5α-Tetrahydroprogesterone 5α-Pregnan-3α-ol-20-one 5alpha-Pregnan-3alpha-ol-20-one (3α)-Allopregnanolone LJPC-0712 |
| Description | Allopregnanolone is a progesterone metabolite. Allopregnanolone is an allosteric modulator of the GABA receptor. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| In Vitro | Allopregnanolone induces a significant increase in proliferation of neuroprogenitor cells derived from the rat hippocampus and human neural stem cells derived from the cerebral cortex in a dose-dependent manner. Allopregnanolone increases the expression of genes that promote mitosis and inhibits the expression of genes that repress cell proliferation[1]. Its biosynthesis begins with progesterone, which is converted to dihydroprogesterone by the enzyme 5α-DHP and after that, the enzyme 3α-HSOR catalyses the reduction of dihydroprogesterone toward allopregnanolone[2] |
| In Vivo | Allopregnanolone increases both the K+-evoked [3H]-glutamate and [3H]-GABA release in P rats. The neurosteroid also increases the basal release of [3H]-glutamate in VO rats in an effect that is dependent on the modulation of NMDA receptors as is reverted by Mg2+[2]. At therapeutic doses by either subcutaneous or intravenous routes, allopregnanolone mouse plasma levels range between 34-51ng/mL by 30min[3]. Allopregnanolone-induced neurogenesis correlates with restoration of learning and memory function in a mouse model of Alzheimer's disease and is comparably efficacious in aged normal mice[4]. Progesterone and allopregnanolone has shown neuroprotective effects in different experimental models including stroke and spinal cord injury[5]. |
| Animal Admin | Rats: Allopregnanolone is dissolved in propylenglycol to a concentration of 0.6 mM and diluted in Krebs Ringer bicarbonate glucose (KRBG) Mg2+-free buffer at pH 7.4 to 120 nM. To antagonize GABA receptors, 120 nM allopregnanolone plus 9.8 μM Bic (Bic+Allo groups) or 9.8 μM Bic alone (Bic groups) is used[2]. Mice: Allopregnanolone is dissolved in 20%w/v HBCD solution at 2.5 mg/mL by brief sonication and is subcutaneously (SC) injected to mice at 0.5, 1, and 10 mg/kg. Additionally, allopregnanolone is dissolved in 6%w/v SBECD solution at 0.5 mg/mL and injected IV to mice at 0.1, 0.5, and 1 mg/kg. HBCD or SBECD alone are included as vehicle controls. Topical transdermal is applied on the shaved dorsal surface at 50mg/kg using a gel solution of 3.3% allopregnanolone (w/w), 45% DMSO, 30% EtOH, 2.5% Klucel MF, 19.2% PEG-300. Intranasal formulations are prepared in both 100% castor oil and 20% HBCD. Intramuscular formulation is administered to mice as allopregnanolone 1.5 mg/mL in 6% SBECD[3]. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 431.2±18.0 °C at 760 mmHg |
| Melting Point | 176-178° |
| Molecular Formula | C21H34O2 |
| Molecular Weight | 318.493 |
| Flash Point | 183.9±13.8 °C |
| Exact Mass | 318.255890 |
| PSA | 37.30000 |
| LogP | 4.89 |
| Appearance | White solid |
| Vapour Pressure | 0.0±2.3 mmHg at 25°C |
| Index of Refraction | 1.524 |
| Storage condition | Store at RT |
| Water Solubility | chloroform: 20 mg/mL, clear, colorless |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Safety Phrases | 22-24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | TU4383000 |