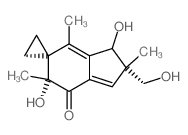
158440-71-2
| Name | (5'R)-5'-hydroxy-1'-(hydroxymethyl)-2',5',7'-trimethylspiro[cyclopropane-1,6'-indene]-4'-one |
|---|---|
| Synonyms |
HMAF
Spiro(cyclopropane-1,5'(5H)-inden)-7'(6'H)-one,6'-hydroxy-3'-(hydroxymethyl)-2',4',6'-trimethyl-,(R) [14C]-Irofulven 6-hydroxymethylacylfulvene (-)-irofulven Acylfulvene,6-(hydroxymethyl) MGI 114 |
| Description | (-)-Irofulven (MGI 114), an Illudin S analog, is a DNA alkylating agent. (-)-Irofulven inhibits the replication of DNA, induces tumor cells apoptosis, and has potent antitumor activity[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | (-)-Irofulven (2.8 μM; 1 hour) induces p53-dependent cell cycle arrest[1]. (-)-Irofulven (0.8 μM, 0.9 μM and 2.8 μM; 1 hour) induces CHK2 activation is related to p53 status in cells[1]. (-)-Irofulven inhibits DNA replication and induces chromosome aberrations (breaks and radials)[1]. Cell Cycle Analysis[1] Cell Line: A2780, CAOV3 and HCT116 cells Concentration: 2.8 μM Incubation Time: 1 hour Result: p53 wild-type cells mainly arrested at G1/S phases, while p53-mutated or p53-null cells arrested at S and G2/M phases. Western Blot Analysis[1] Cell Line: A2780, CAOV3 and HCT116 cells Concentration: 0.8 μM, 0.9 μM and 2.8 μM Incubation Time: 1 hour Result: Induced the Thr 68 phosphorylation of CHK2 kinase in cells. |
| In Vivo | (-)-Irofulven (7 mg/kg; i.p; on days 1-5 and 8-12) produces a statistically significant increase in the median survival of mice bearing tumor cells[2]. Animal Model: Male and female athymic BALB/c mice (nu/nu genotype, 6 weeks old or older) injected with human glioblastoma multiforme[2]. Dosage: 7 mg/kg Administration: i.p; on days 1-5 and 8-12 Result: Was active against all tumor lines. |
| References |
| Density | 1.28g/cm3 |
|---|---|
| Boiling Point | 501ºC at 760 mmHg |
| Molecular Formula | C15H18O3 |
| Molecular Weight | 246.30200 |
| Flash Point | 270.9ºC |
| Exact Mass | 246.12600 |
| PSA | 57.53000 |
| LogP | 1.66560 |
| Vapour Pressure | 3.9E-12mmHg at 25°C |
| Index of Refraction | 1.617 |
| Storage condition | -20°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
|
~75% 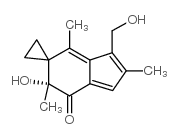
158440-71-2 |
| Literature: McMorris, Trevor C.; Hu, Yi; Yu, Jian; Kelner, Michael J. Chemical Communications, 1997 , # 3 p. 315 - 316 |
|
~% 
158440-71-2 |
| Literature: Journal of Labelled Compounds and Radiopharmaceuticals, , vol. 41, # 4 p. 279 - 285 |
| Precursor 3 | |
|---|---|
| DownStream 0 | |