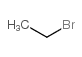
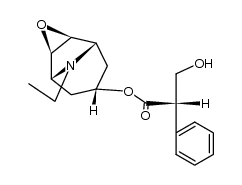
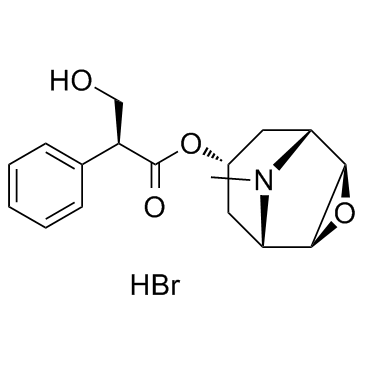
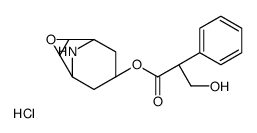
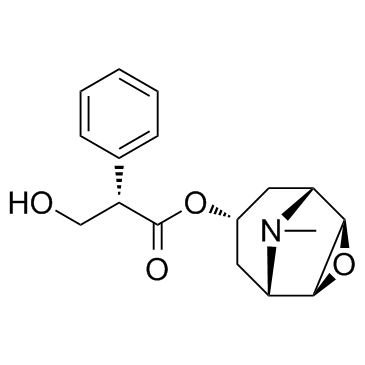
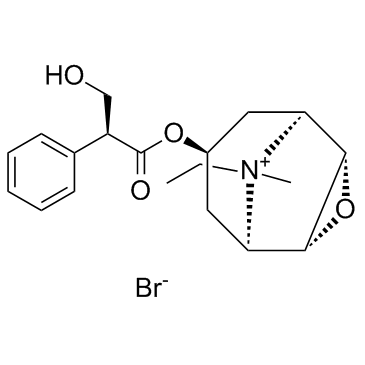
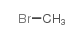30286-75-0
| Name | Tersigat |
|---|---|
| Synonyms |
Oxivent
Oxitropium Oxitropium bromide Ba-253-BR-L Ventilat oxytropium bromide Bromure d'oxitropium |
| Description | Oxitropium bromide is an mAChR antagonist used as an anticholinergic bronchodilator drug for the treatment of asthma and chronic obstructive pulmonary disease. |
|---|---|
| Related Catalog | |
| Target |
mAChR[1] |
| In Vitro | Oxitropium bromide is a muscarinic antagonist which blocks musucarinic acetylcholine receptors (mAChR). Incubation with oxitropium bromide of untreated diaphragm muscle and diaphragm muscle injected with endotoxin does not increase the force-frequency curves dose-dependently in vitro; however, it causes both types of muscle to be fatigue resistant[1]. |
| In Vivo | Oxitropium bromide inhalation shifts force-frequency curves upward at 2 h after inhalation and inhibits the decrease of force-frequency curves due to endotoxin injection in vivo[1]. Oxitropium bromide strongly and persistently inhibits the acetylcholine (ACh)-induced resistance. The increase in resistance induced by histamine, serotonin, leukotriene D4 or antigen is prevented by oxitropium bromide oxitropium bromide[2]. Inhalation of the anticholinergic agent oxitropium bromide at doses of 1.5 μg and higher greatly attenuates the decrease in mucus score produced by intravenous histamine but not by inhaled histamine[3]. |
| Animal Admin | Mice: In the oxitropium bromide inhalation group, animals are given 2 puffs of inhalation from a oxitropium bromide MDI (metered dose inhaler) via a 75-mL spacer, and then diaphragm muscles are dissected and measured as to contractility immediately, 1 hour, 2 hours and 4 hours later (n=5 animals each). An animal is placed in a centrifugal tube (inner diameter=30 mm) with a round hole (diameter=10 mm) in the bottom, its nose and mouth being exposed through the hole to breath. An oxitropium bromide MDI (metered dose inhaler) releases 2 puffs into a spacer attached to the tube. Aerosols of oxitropium bromide are inhaled for about 10 seconds, while the animal is breathing spontaneously through the hole of the tube[1]. |
| References |
| Melting Point | 203-204° (dec) |
|---|---|
| Molecular Formula | C19H26BrNO4 |
| Molecular Weight | 412.31800 |
| Exact Mass | 411.10500 |
| PSA | 59.06000 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
|
~% 
30286-75-0 |
| Literature: Journal of the American Chemical Society, , vol. 78, p. 3448,3452 |
|
~84% 
30286-75-0 |
| Literature: Banholzer; Pook Arzneimittel-Forschung/Drug Research, 1985 , vol. 35, # 1 A p. 217 - 228 |
|
~% 
30286-75-0 |
| Literature: Arzneimittel-Forschung/Drug Research, , vol. 35, # 1 A p. 217 - 228 |
|
~% 
30286-75-0 |
| Literature: Arzneimittel-Forschung/Drug Research, , vol. 35, # 1 A p. 217 - 228 |
| Precursor 5 | |
|---|---|
| DownStream 2 | |