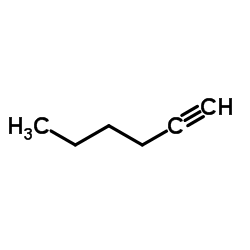
99107-52-5
| Name | 2-Butyl-4-methoxy-1-naphthyl acetate |
|---|---|
| Synonyms |
Bunamiodilo
Bunamiodylum Bunamijodylum BUNAMIODYL 1-acetoxy-2-butyl-4-methoxynaphthalene bunaprolast Bunamiodylsaeure 3-(3-Butylamido-2,4,6-triiodphenyl)-2-ethylacrylsaeure 2-(3-Butyramido-3,4,6-triiodophenylmethylene)butyric acid Buniodyl Bunamiodylum [INN-Latin] 2-(3-Butyrylamino-2,4,6-triiod-benzyliden)-buttersaeure Bunamiodilo [INN-Spanish] 1-acetoxy-2-n-butyl-4-methoxy-naphthalene U66858 |
| Description | U66858 is a potent inhibitor of LTB4 production in human whole blood. U66858 also exhibits significant inhibition of lipoxygenase and TXB2 release. |
|---|---|
| Related Catalog | |
| Target |
LTB4 TXB2 5-Lipoxygenase |
| In Vitro | U66858 (U-66,858) undergoes deacetylation to an initial metabolite with similar pharmacological potency. The inhibitory effects of the semi-quinone U66858 and its metabolite U-68,244 on the ionophore-induced formation of leukotriene B4 (LTB4) are examined in human whole blood (WB). Preincubation of U-66858 and U-68,244 for 1 min prior to challenge of blood with calcium ionophore A23187 results in IC50s of 1080±644 and 820±442 nM, respectively. After 60 min preincubation, IC50s are 250±85 and 270±79 nM. The activity of the lipoxygenase inhibitor AA-861 in this system is similar to that of U-66858, while vitamin K and the sulphate conjugate of U-66858 show significant inhibition of LTB4 release only at micromolar concentrations. U-66858 exhibits significant inhibition of thromboxane A2 release (p<0.02) in a comparative study with the known cyclooxygenase (CO) inhibitor Flurbiprofen[1]. |
| In Vivo | The IgE-mediated hypersensitivity to Ascaris antigen in reactor rhesus primates is used to assess the pharmacologic profile of U66858 (U-66,858). When U-66858 is given by the oral route, it shows dose-related inhibition of resistance (RL) and compliance (Cdyn) changes. When U-66858 is given by the aerosol route, it shows dose independent inhibition. In 15 animals, aerosols (52±32 to 53±10% for RL, p=0.05 and 45±19 to 28±19% Cdyn inhibitions, p=0.05) for 5.0-0.1% aerosol. By the oral route, inhibition is seen at 1-4 h following administration. In 5 animals, oral doses of 10 and 5 mg/kg inhibit (RL by 98±2 to 78±1.5%, p=0.01 and Cdyn by 75±17 to 60.9±9.1%, p=0.05) by 10 and 5 mg/kg U-66858, respectively[2]. |
| References |
| Density | 1.084g/cm3 |
|---|---|
| Boiling Point | 413ºC at 760mmHg |
| Molecular Formula | C17H20O3 |
| Molecular Weight | 272.33900 |
| Flash Point | 175.3ºC |
| Exact Mass | 272.14100 |
| PSA | 35.53000 |
| LogP | 4.11630 |
| Index of Refraction | 1.558 |
| HS Code | 2915390090 |
|---|
|
~81% 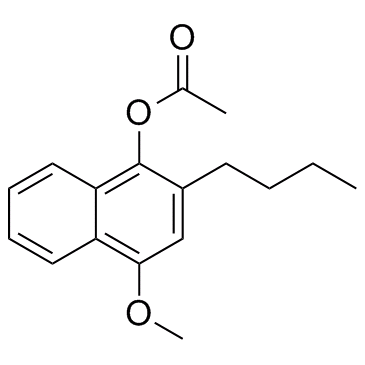
99107-52-5 |
| Literature: Yamashita; Schaub; Back; White; Toy; Ghazal; Burdick; Brashler; Holm Journal of Medicinal Chemistry, 1990 , vol. 33, # 2 p. 775 - 781 |
|
~63% 
99107-52-5 |
| Literature: Yamashita, A.; Timko, J.M.; Watt, W. Tetrahedron Letters, 1988 , vol. 29, # 21 p. 2513 - 2516 |
|
~66% 
99107-52-5 |
| Literature: Yamashita, A.; Timko, J.M.; Watt, W. Tetrahedron Letters, 1988 , vol. 29, # 21 p. 2513 - 2516 |
| HS Code | 2915390090 |
|---|---|
| Summary | 2915390090. esters of acetic acid. VAT:17.0%. Tax rebate rate:13.0%. Supervision conditions:AB(certificate of inspection for goods inward,certificate of inspection for goods outward). MFN tariff:5.5%. General tariff:30.0% |