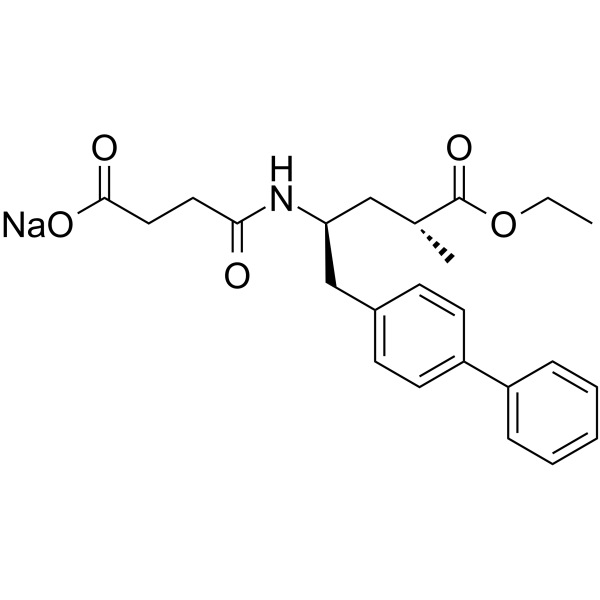
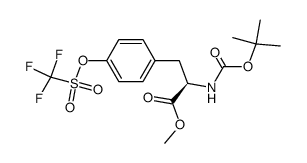
149709-62-6
| Name | Sacubitril |
|---|---|
| Synonyms |
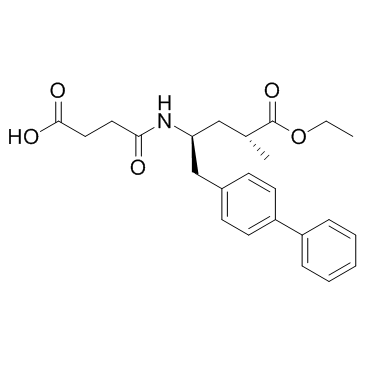
4-[[(2S,4R)-5-ethoxy-4-methyl-5-oxo-1-(4-phenylphenyl)pentan-2-yl]amino]-4-oxobutanoic acid
AHU-377 ENTRESTO 4-(((2S,4R)-1-([1,1'-biphenyl]-4-yl)-5-ethoxy-4-methyl-5-oxopentan-2-yl)amino)-4-oxobutanoic acid 4-{[(2S,4R)-1-(4-Biphenylyl)-5-ethoxy-4-methyl-5-oxo-2-pentanyl]amino}-4-oxobutanoic acid UNII-17ERJ0MKGI AHU377 Sacubitril |
| Description | Sacubitril (AHU-377) is a potent NEP inhibitor with an IC50 of 5 nM. Sacubitril (AHU-377) is a component of the heart failure medicine LCZ696. |
|---|---|
| Related Catalog | |
| Target |
IC50: 5 nM (NEP)[1] |
| In Vitro | Sacubitril (AHU-377) is a single molecule that is comprised of molecular moieties of valsartan, an ARB, and Sacubitril (AHU-377), a neprilysin inhibitor (1:1 ratio). Sacubitril (AHU-377) is converted by enzymatic cleavage of the ethyl ester into the active neprilysin inhibiting metabolite LBQ657[2]. The inactive NEPi precursor, Sacubitril (AHU-377), does not inhibit collagen accumulation in fibroblasts nor cardiac myocyte hypertrophy. In cardiac fibroblasts, the active NEPi LBQ657 had no discernible effects. In contrast, LBQ657 modestly inhibits cardiac myocyte hypertrophy[3]. |
| In Vivo | In humans, Sacubitril (AHU-377) (tmax 0.5-1.1 h) are absorbed quickly. Sacubitril (AHU-377) is converted rapidly into LBQ657 with its tmax being reached in 1.9-3.5 h. Mean t1/2 values for the biologically active LBQ657 is 9.9-11.1 h[2]. In vehicle-treated dogs, ANF increases urinary sodium excretion from 17.3±3.6 to 199.5±18.4 pequivkglmin. This effect is potentiated significantly in animals which receive Sacubitril (AHU-377). Urinary volume is also potentiated in animals which receive an iv administration of Sacubitril (AHU-377)[1]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 656.9±55.0 °C at 760 mmHg |
| Molecular Formula | C24H29NO5 |
| Molecular Weight | 411.491 |
| Flash Point | 351.1±31.5 °C |
| Exact Mass | 411.204559 |
| PSA | 96.19000 |
| LogP | 3.96 |
| Vapour Pressure | 0.0±2.1 mmHg at 25°C |
| Index of Refraction | 1.549 |
|
~% 
149709-62-6 |
| Literature: NOVARTIS AG Patent: WO2008/83967 A2, 2008 ; Location in patent: Page/Page column 148-149 ; |
|
~% 
149709-62-6 |
| Literature: Ksander, Gary M.; Ghai, Raj D.; deJesus, Reynalda; Diefenbacher, Clive G.; Yuan, Andrew; et al. Journal of Medicinal Chemistry, 1995 , vol. 38, # 10 p. 1689 - 1700 |
|
~% 
149709-62-6 |
| Literature: Ksander, Gary M.; Ghai, Raj D.; deJesus, Reynalda; Diefenbacher, Clive G.; Yuan, Andrew; et al. Journal of Medicinal Chemistry, 1995 , vol. 38, # 10 p. 1689 - 1700 |
|
~% 
149709-62-6 |
| Literature: Ksander, Gary M.; Ghai, Raj D.; deJesus, Reynalda; Diefenbacher, Clive G.; Yuan, Andrew; et al. Journal of Medicinal Chemistry, 1995 , vol. 38, # 10 p. 1689 - 1700 |
|
~% 
149709-62-6 |
| Literature: Ksander, Gary M.; Ghai, Raj D.; deJesus, Reynalda; Diefenbacher, Clive G.; Yuan, Andrew; et al. Journal of Medicinal Chemistry, 1995 , vol. 38, # 10 p. 1689 - 1700 |
| Precursor 4 | |
|---|---|
| DownStream 0 | |