87233-61-2
| Name | emedastine |
|---|---|
| Synonyms |
Emedastine
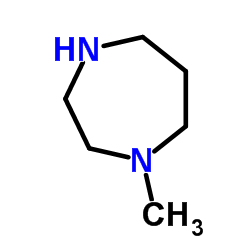
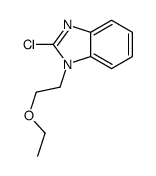
1-(2-ethoxyethyl)-2-(4-methyl-1,4-diazepan-1-yl)-1H-benzo[d]imidazole Emadine Emedastina [INN-Spanish] AL 3432A Emedastina 1-methyl-4-(1-(2-ethoxyethyl)-1H-benzimidazol-2-yl)[1,4]diazepane 1-(2-ethoxyethyl)-2-(4-methyl-1,4-diazepan-1-yl)benzimidazole 1-(2-Ethoxyethyl)-2-(4-methyl-1,4-diazepan-1-yl)-1H-benzimidazole emedastinum [INN_la] Rapimine 1-(2-Ethoxyethyl)-2-(hexahydro-4-methyl-1H-1,4-diazepin-1-yl)-1H-benzimidazole 1-[2-(Ethoxy)ethyl]-2-(4-methyl-1-homopiperazinyl)benzimidazole Emedastine [INN] Emedastinum UNII-9J1H7Y9OJV MFCD00865647 Emedastinum [INN-Latin] |
| Description | Emedastine is an orally active, selective and high affinity histamine H1 receptor antagonist with a Ki value of 1.3 nM. Emedastine is a benzimidazole derivative with potent antiallergic properties and used for allergic rhinitis, allergic skin diseases and allergic conjunctivitis[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
H1 Receptor:1.3 nM (Ki) H2 Receptor:49067 nM (Ki) H3 Receptor:12430 nM (Ki) |
| In Vitro | Emedastine inhibits histamine H2 receptor (Ki=49067 nM) and histamine H3 receptor (Ki=12430 nM)[1]. High concentrations of Emedastine (1 and 10 ng/ml) significantly inhibits type 1 collagen production in normal human dermal fibroblasts[2]. Emedastine (1, 10, 100, 1000 nM) at concentrations of ≥ 10 nM inhibits CC chemokine-elicited eosinophil migration[2]. |
| In Vivo | Emedastine (0.03, 0.1, 0.3 mg/kg; orally; pretreatment of 30 min) significantly suppresses histamine-induced scratching with 0.1 and 0.3 mg/kg but not 0.03 mg/kg[3]. Pretreatment with Emedastine (0.03, 0.1, 0.3 mg/kg; orally) significantly inhibits the scratching induced by substance P and leukotriene B[3]. Emedastine (0.3 mg/kg, p.o.) produces significant inhibition of passive peritoneal anaphylaxis in guinea-pigs[2]. Emedastine inhibits histamine-induced contractions of isolated ileum (IC50=6.1 nM)[2]. Animal Model: Male ICR mice 5-6 weeks of age[3] Dosage: 0.03, 0.1, 0.3 mg/kg Administration: Orally; 30 min before pruritogen injection Result: Significantly suppressed histamine-induced scratching with pretreatment of 0.1 and 0.3 mg/kg. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 446.6±55.0 °C at 760 mmHg |
| Melting Point | 148-151ºC |
| Molecular Formula | C17H26N4O |
| Molecular Weight | 302.414 |
| Flash Point | 223.9±31.5 °C |
| Exact Mass | 302.210663 |
| PSA | 33.53000 |
| LogP | 3.02 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.595 |
| Storage condition | -20℃ |
| HS Code | 2933990090 |
|---|
|
~80% 
87233-61-2 |
| Literature: Iemura; Kawashima; Fukuda; Ito; Tsukamoto Journal of Medicinal Chemistry, 1986 , vol. 29, # 7 p. 1178 - 1183 |
|
~% 
87233-61-2 |
| Literature: Journal of Medicinal Chemistry, , vol. 29, # 7 p. 1178 - 1183 |
|
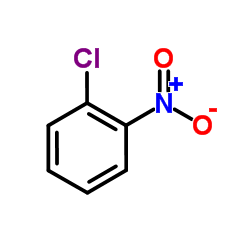
~% 
87233-61-2 |
| Literature: Journal of Medicinal Chemistry, , vol. 29, # 7 p. 1178 - 1183 |
|
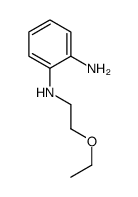
~% 
87233-61-2 |
| Literature: Journal of Medicinal Chemistry, , vol. 29, # 7 p. 1178 - 1183 |
|
~% 
87233-61-2 |
| Literature: Journal of Medicinal Chemistry, , vol. 29, # 7 p. 1178 - 1183 |
| Precursor 6 | |
|---|---|
| DownStream 0 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |