42830-48-8
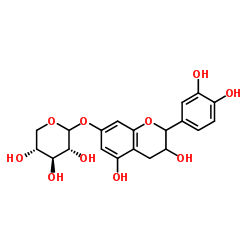
| Name | (2S,3R,4S,5R)-2-[[(2R,3S)-2-(3,4-dihydroxyphenyl)-3,5-dihydroxy-3,4-dihydro-2H-chromen-7-yl]oxy]oxane-3,4,5-triol |
|---|---|
| Synonyms |
2-(3,4-Dihydroxyphenyl)-3,5-dihydroxy-3,4-dihydro-2H-chromen-7-yl D-xylopyranoside
D-Xylopyranoside, 2-(3,4-dihydroxyphenyl)-3,4-dihydro-3,5-dihydroxy-2H-1-benzopyran-7-yl (2R,3S)-2-(3,4-Dihydroxyphenyl)-3,5-dihydroxy-3,4-dihydro-2H-chromen-7-yl β-D-xylopyranoside β-D-Xylopyranoside, (2R,3S)-2-(3,4-dihydroxyphenyl)-3,4-dihydro-3,5-dihydroxy-2H-1-benzopyran-7-yl |
| Description | Catechin-7-O-β-D-xylopyranoside is an antioxidant compound with strong DPPH free radical scavenging ability. Catechin-7-O-β-D-xylopyranoside can be extracted from birch inner bark and nepeta stem bark[1][2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.7±0.1 g/cm3 |
|---|---|
| Boiling Point | 773.8±60.0 °C at 760 mmHg |
| Molecular Formula | C20H22O10 |
| Molecular Weight | 422.38 |
| Flash Point | 421.8±32.9 °C |
| Exact Mass | 422.121307 |
| PSA | 169.30000 |
| LogP | -0.35 |
| Vapour Pressure | 0.0±2.8 mmHg at 25°C |
| Index of Refraction | 1.735 |
| Hazard Codes | Xi |
|---|