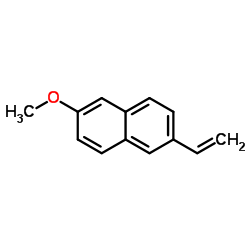
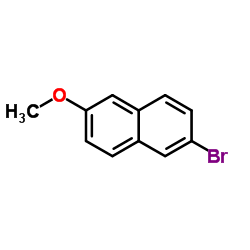
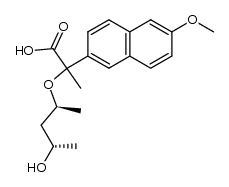
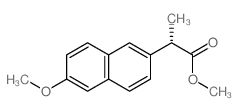
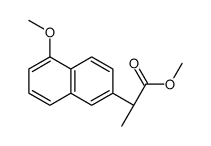
23979-41-1
| Name | (r)-naproxen |
|---|---|
| Synonyms |
piproxen
(+)-(S)-Naproxen Anaprox (R)-Naproxen MFCD00870716 (2S)-2-(6-methoxynaphthalen-2-yl)propanoic acid (2R)-2-(6-Methoxy-2-naphthyl)propanoic acid NAPRELAN (2S)-2-(6-Methoxy-2-naphthyl)propanoic acid (2S)-2-(6-Methoxynaphth-2-yl)propanoic acid Naproxen Apronax EINECS 245-966-6 (+)-NAPROXEN (S)-(+)-6-methoxy-α-methyl-2-naphthaleneacetic acid (S)-(+)-Naproxen ALEVE (S)-naproxen Naprosyn |
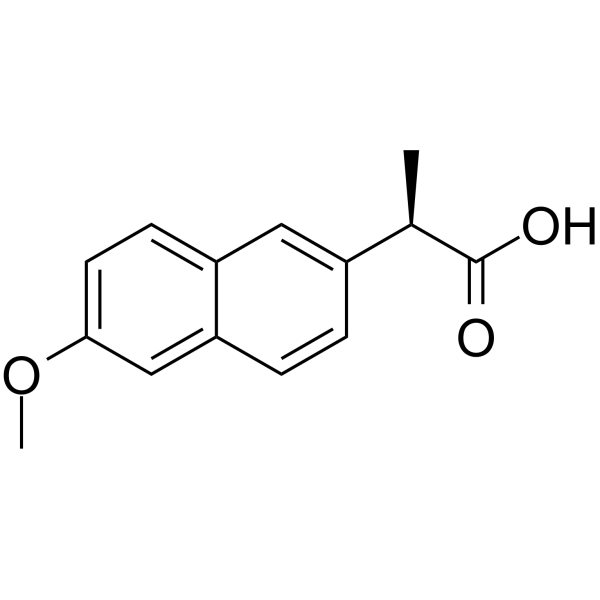
| Description | l-Naproxen ((R)-Naproxen) is an enantiomer of (S)-Naproxen. l-Naproxen can inhibit Cdc42 and Rac1 (EC50=96 μM and 212 μM, respectively), and show anti-tumor activity[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | l-Naproxen (30-1000 μM; 2 h) inhibits Rac1 and Cdc42 activity selectively in cells[3]. l-Naproxen (0-300 μM; 48 h) inhibits migration of immortalized human ovarian cancer cells[3]. l-Naproxen (300 μM; 48 h) shows activity via a COX-independent mechanism[3]. Cell Viability Assay[3] Cell Line: HeLa cells Concentration: 30-1000 μM Incubation Time: 2 hour Result: Inhibited Rac1 and Cdc42 activity in a dose dependent manner with the EC50 values of 212 μM and 96 μM, respectively. Cell Migration Assay [3] Cell Line: OvCa429 and OvCa433 cells Concentration: 0-300 μM Incubation Time: 48 hour Result: Had a statistically significant inhibitory effect at 300 μM compared to untreated controls. Western Blot Analysis[3] Cell Line: OvCa433 cells Concentration: 300 μM Incubation Time: 48 hour Result: Exhibited higher levels of phosphorylated EGFR (pEGFR) and (pERK) compared to unstimulated controls. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 403.9±20.0 °C at 760 mmHg |
| Melting Point | 156-158ºC(lit.) |
| Molecular Formula | C14H14O3 |
| Molecular Weight | 230.259 |
| Flash Point | 154.5±15.3 °C |
| Exact Mass | 230.094299 |
| PSA | 46.53000 |
| LogP | 3.00 |
| Vapour Pressure | 0.0±1.0 mmHg at 25°C |
| Index of Refraction | 1.609 |
| Storage condition | 2-8°C |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn |
| Risk Phrases | R22 |
| Safety Phrases | S36/S37 |
| RIDADR | UN 2811 6.1/PG 3 |
| WGK Germany | 3.0 |
| HS Code | 2918990090 |
| Precursor 9 | |
|---|---|
| DownStream 2 | |
| HS Code | 2918990090 |
|---|---|
| Summary | 2918990090. other carboxylic acids with additional oxygen function and their anhydrides, halides, peroxides and peroxyacids; their halogenated, sulphonated, nitrated or nitrosated derivatives. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |