544-64-9
| Name | Myristoleic acid |
|---|---|
| Synonyms |
cis-9-tetradecenoic acid
cis-δ(9)-tetradecenoic acid (9Z)-9-Tetradecenoic acid Myristoleic acid (14:1(n-5)) tetradecenoic acid (9Z)-Tetradec-9-enoic acid Z-9-tetradecenoic acid (9Z)-Tetradecenoic acid Z-9-octadecene-4-olide MFCD00004436 9Z-tetradecenoic acid Myristoleate EINECS 208-876-8 Myristoleic acid |
| Description | Myristoleic acid, a cytotoxic component in the extract from Serenoa repens, induces apoptosis and necrosis in human prostatic LNCaP cells[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Myristoleic acid induces both apoptosis (100 μg/mL, 89.5%) and necrosis (100 μg/mL, 81.8%) in LNCaP cells[1]. Myristoleic acid inhibited RANKL-induced osteoclast formation in vitro, especially, at later stages of differentiation[2]. Cell Proliferation Assay[1] Cell Line: Human prostatic carcinoma LNCaP cells. Concentration: 0, 50, 100, 150, 200, 250 μg/mL. Incubation Time: 24 h. Result: When LNCaP cells were treated with 130 μg/mL extract or 100 μg/mL myristoleic acid for 24 hr, the proportion of apoptotic cells was 16.5 and 8.8%, and that of necrotic one was 46.8 and 81.8%, respectively. |
| In Vivo | Myristoleic acid (2 mg/kg, IP every 24 h for 4 days) prevents RANKL-induced bone loss and osteoclast formation in mice[2]. Animal Model: C57BL/6 mice at 5 weeks[2]. Dosage: 0.2, 2 mg/kg Administration: IP every 24 h for 4 days. Result: Co-administration of myristoleic acid suppressed generation of TRAP-positive osteoclasts induced by sRANKL and attenuated the increases in osteoclastic indices of Oc.S/BS, N.Oc/B. Pm and ES/BS in a dose-dependent manner. |
| References |
| Density | 0.9±0.1 g/cm3 |
|---|---|
| Boiling Point | 338.9±0.0 °C at 760 mmHg |
| Melting Point | -4.5--4ºC(lit.) |
| Molecular Formula | C14H26O2 |
| Molecular Weight | 226.355 |
| Flash Point | 206.5±14.4 °C |
| Exact Mass | 226.193283 |
| PSA | 37.30000 |
| LogP | 5.57 |
| Appearance | Liquid |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.465 |
| Storage condition | −20°C |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | Eyeshields;full-face respirator (US);Gloves;multi-purpose combination respirator cartridge (US);type ABEK (EN14387) respirator filter |
| Hazard Codes | Xi |
| Risk Phrases | 36/37/38 |
| Safety Phrases | 26-36 |
| RIDADR | NA 1993 / PGIII |
| WGK Germany | 3 |
| HS Code | 2916190090 |
|
~77% 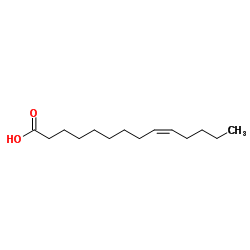
544-64-9 |
| Literature: Journal of Pharmaceutical Sciences, , vol. 83, # 3 p. 296 - 299 |
|
~% 
544-64-9 |
| Literature: Journal of Chemical Ecology, , vol. 21, # 10 p. 1495 - 1510 |
|
~% 
544-64-9 |
| Literature: Journal of the Chemical Society, , p. 775 - 778 |
|
~% 
544-64-9 |
| Literature: Journal of the Chemical Society, , p. 775 - 778 |
|
~% 
544-64-9 |
| Literature: Journal of the Chemical Society, , p. 775 - 778 |
|
~% 
544-64-9 |
| Literature: Journal of the Chemical Society, , p. 671,674 |
|
~% 
544-64-9 |
| Literature: Chemische Berichte, , vol. 108, # 11 p. 3582 - 3595 |
|
~% 
544-64-9 |
| Literature: Chemische Berichte, , vol. 108, # 11 p. 3582 - 3595 |
| Precursor 8 | |
|---|---|
| DownStream 3 | |
| HS Code | 2916190090 |
|---|---|
| Summary | 2916190090 unsaturated acyclic monocarboxylic acids, their anhydrides, halides, peroxides, peroxyacids and their derivatives。supervision conditions:AB(certificate of inspection for goods inward,certificate of inspection for goods outward)。VAT:17.0%。tax rebate rate:9.0%。MFN tariff:6.5%。general tariff:30.0% |