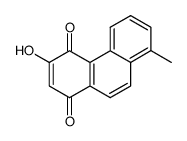
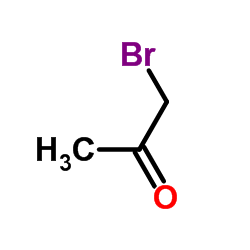
568-73-0
| Name | Tanshinone I |
|---|---|
| Synonyms |
Salvia quinone
Tanshinone Tanshinon I tanshinone-I Tanshinone 1 1,6-Dimethylphenanthro[1,2-b]furan-10,11-dione TANSHINONES IIA Tanshinquinone I Tanshine I MFCD00238692 |
| Description | Tanshinone I is an inhibitor of type IIA human recombinant sPLA2 (IC50=11 μM) and rabbit recombinant cPLA2 (IC50=82 μM). |
|---|---|
| Related Catalog | |
| Target |
IC50: 11 μM (sPLA2), 82 μM (cPLA2)[1]. |
| In Vitro | Tanshinone I inhibits PGE2 formation from LPS-induced RAW macrophages (IC50=38 μM). When Tanshinone I is added simultaneously with LPS, this compound clearly inhibits PGE2 production (IC50=38 μM) at 10-100 μM. Tanshinone I also reduces PGE2 production (IC50=46 μM) when added after COX-2 is fully induced. The fact that Tanshinone I inhibits PGE2 production by pre-induced COX-2 strongly suggests that this compound may directly inhibit COX-2 activity and/or affect PLA2 activity. When Tanshinone I is incubated with two different forms of phospholipase A2 (PLA2), it clearly inhibits sPLA2 (IC50=11 μM) in a concentration-dependent manner. Although being less potent, Tanshinone I also inhibits cPLA2 (IC50=82 μM)[1]. |
| In Vivo | Tanshinone I shows antiinflammatory activity in rat carrageenan-induced paw oedema and adjuvant-induced arthritis. In order to establish the anti-inflammatory activity of Tanshinone I, the classical animal models of acute and chronic inflammation [rat carrageenan (CGN)-induced paw oedema and rat adjuvant-induced arthritis (AIA)] are employed. When Tanshinone I is orally administered, it shows significant anti-inflammatory activity against CGN-induced paw oedema (47% inhibition at 160 mg/kg), while the IC50 of indomethacin is 7.1 mg/kg. In AIA, Tanshinone I gives 27% inhibition of secondary inflammation at 18 day with an oral dose of 50 mg/kg/day, whereas prednisolone (5 mg/kg/day) shows potent inhibition (65%)[1]. |
| Kinase Assay | As sources of PLA2, human recombinant sPLA2 (type IIA) is purified from CHO cells transfected with the PLA2 gene and rabbit recombinant platelet cPLA2 is obtained through its expression in baculovirus. The standard reaction mixture (200 μL) contained 100 mM Tris-HCl buffer (pH 9.0) with 6 mM CaCl2 and 20 nmol 1-acyl-[1-14C]-arachidonyl-sn-glycerophosphoethanolamine (2000 cpm/nmol) in the presence or absence of Tanshinone I. The reaction is started by adding 50 ng purified sPLA2 or cPLA2. After 20 min at 37°C, the free fatty acid generated is analysed. Under these standard conditions, the reaction mixture in the absence of Tanshinone I released approximately 10% of free fatty acid from the phospholipid substrate added[1]. |
| Cell Assay | RAW 264.7 cells are cultured with DMEM supplemented with 10% FBS and 1% antibiotics under 5% CO2 at 37°C. Briefly, cells are plated in 96-well plates (2×105 cells/well). LPS (1 ug/mL) and Tanshinone I are simultaneously added and incubated for 24 h, unless otherwise specified. The PGE2 concentration in the medium is measured using an EIA kit for PGE2. In order to determine the effects of Tanshinone I on PGE2 production after induction of COX-2, cells are incubated with LPS (1 ug/mL) for 24 h and thoroughly washed. Then, Tanshinone I is added without LPS and the cells are incubated for another 24 h. From the medium, PGE2 concentrations are measured. The cytotoxicity of Tanshinone I to RAW cells is checked using the MTT assay. Tanshinone I does not show any cytotoxicity up to 100 uM[1]. |
| Animal Admin | Mice[1] In order to evaluate the inhibitory activity of Tanshinone I against animal models of acute and chronic inflammation, rat carrageenan (CGN)-induced paw oedema and adjuvant-induced arthritis (AIA) models are employed. Briefly, 1% CGN dissolved in pyrogen-free saline (0.05 mL) is injected into right hind paw of rats for the paw oedema test. After 5 h, swelling of the treated paw is measured using a plethysmometer. Tanshinone I dissolved in 0.5% CMC is administered orally 1 h prior to CGN injection. For the AIA test, an arthritic inflammation is provoked by injection of Mycobacterium Butyricum (0.6 mL/rat) dissolved in mineral oil to the right hind paw of rats. Tanshinone I is orally administered every day. The swelling of the treated and untreated paws is measured using a plethysmometer. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 498.0±24.0 °C at 760 mmHg |
| Melting Point | 233-234ºC |
| Molecular Formula | C18H12O3 |
| Molecular Weight | 276.286 |
| Flash Point | 245.9±15.6 °C |
| Exact Mass | 276.078644 |
| PSA | 47.28000 |
| LogP | 4.44 |
| Vapour Pressure | 0.0±1.3 mmHg at 25°C |
| Index of Refraction | 1.676 |
| Storage condition | 2-8°C |
|
SECTION 1: Identification of the substance/mixture and of the company/undertaking Product identifiers Product name: Tanshinone I REACH No.: A registration number is not available for this substance as the substance or its uses are exempted from registration, the annual tonnage does not require a registration or the registration is envisaged for a later registration deadline.
CAS-No.: 568-73-0 Relevant identified uses of the substance or mixture and uses advised against Identified uses: Laboratory chemicals, Manufacture of substances SECTION 2: Hazards identification Classification of the substance or mixture Classification according to Regulation (EC) No 1272/2008 Chronic aquatic toxicity (Category 4), H413 For the full text of the H-Statements mentioned in this Section, see Section 16. Not a hazardous substance or mixture according to EC-directives 67/548/EEC or 1999/45/EC. Label elements Labelling according Regulation (EC) No 1272/2008 Pictogramnone Signal wordnone Hazard statement(s) H413May cause long lasting harmful effects to aquatic life. Precautionary statement(s) none Supplemental Hazardnone Statements Other hazards - none SECTION 3: Composition/information on ingredients Substances Formula: C18H12O3 Molecular Weight: 276,29 g/mol CAS-No.: 568-73-0 No components need to be disclosed according to the applicable regulations. For the full text of the H-Statements and R-Phrases mentioned in this Section, see Section 16 SECTION 4: First aid measures Description of first aid measures General advice Consult a physician. Show this safety data sheet to the doctor in attendance. If inhaled If breathed in, move person into fresh air. If not breathing, give artificial respiration. Consult a physician. In case of skin contact Wash off with soap and plenty of water. Consult a physician. In case of eye contact Flush eyes with water as a precaution. If swallowed Never give anything by mouth to an unconscious person. Rinse mouth with water. Consult a physician. Most important symptoms and effects, both acute and delayed The most important known symptoms and effects are described in the labelling (see section 2.2) and/or in section 11 Indication of any immediate medical attention and special treatment needed no data available SECTION 5: Firefighting measures Extinguishing media Suitable extinguishing media Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide. Special hazards arising from the substance or mixture Carbon oxides Advice for firefighters Wear self contained breathing apparatus for fire fighting if necessary. Further information no data available SECTION 6: Accidental release measures Personal precautions, protective equipment and emergency procedures Avoid dust formation. Avoid breathing vapours, mist or gas. Ensure adequate ventilation. For personal protection see section 8. Environmental precautions Prevent further leakage or spillage if safe to do so. Do not let product enter drains. Discharge into the environment must be avoided. Methods and materials for containment and cleaning up Pick up and arrange disposal without creating dust. Sweep up and shovel. Keep in suitable, closed containers for disposal. Reference to other sections For disposal see section 13. SECTION 7: Handling and storage Precautions for safe handling Provide appropriate exhaust ventilation at places where dust is formed.Normal measures for preventive fire protection. For precautions see section 2.2. Conditions for safe storage, including any incompatibilities Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Recommended storage temperature: 2 - 8 °C Store with desiccant. Specific end use(s) Apart from the uses mentioned in section 1.2 no other specific uses are stipulated SECTION 8: Exposure controls/personal protection Control parameters Components with workplace control parameters Exposure controls Appropriate engineering controls Handle in accordance with good industrial hygiene and safety practice. Wash hands before breaks and at the end of workday. Personal protective equipment Eye/face protection Use equipment for eye protection tested and approved under appropriate government standards such as NIOSH (US) or EN 166(EU). Skin protection Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique (without touching glove's outer surface) to avoid skin contact with this product. Dispose of contaminated gloves after use in accordance with applicable laws and good laboratory practices. Wash and dry hands. The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and the standard EN 374 derived from it. Full contact Material: Nitrile rubber Minimum layer thickness: 0,11 mm Break through time: 480 min Material tested:Dermatril® (KCL 740 / Z677272, Size M) Splash contact Material: Nitrile rubber Minimum layer thickness: 0,11 mm Break through time: 480 min Material tested:Dermatril® (KCL 740 / Z677272, Size M) data source: KCL GmbH, D-36124 Eichenzell, phone +49 (0)6659 87300, test method: EN374 If used in solution, or mixed with other substances, and under conditions which differ from EN 374, contact the supplier of the CE approved gloves. This recommendation is advisory only and must be evaluated by an industrial hygienist and safety officer familiar with the specific situation of anticipated use by our customers. It should not be construed as offering an approval for any specific use scenario. Body Protection Choose body protection in relation to its type, to the concentration and amount of dangerous substances, and to the specific work-place., The type of protective equipment must be selected according to the concentration and amount of the dangerous substance at the specific workplace. Respiratory protection Respiratory protection is not required. Where protection from nuisance levels of dusts are desired, use type N95 (US) or type P1 (EN 143) dust masks. Use respirators and components tested and approved under appropriate government standards such as NIOSH (US) or CEN (EU). Control of environmental exposure Prevent further leakage or spillage if safe to do so. Do not let product enter drains. Discharge into the environment must be avoided. SECTION 9: Physical and chemical properties Information on basic physical and chemical properties a) AppearanceForm: solid b) Odourno data available c) Odour Thresholdno data available d) pHno data available e) Melting point/freezingMelting point/range: 229 - 234 °C point f) Initial boiling point and no data available boiling range g) Flash pointno data available h) Evapouration rateno data available i) Flammability (solid, gas) no data available j) Upper/lowerno data available flammability or explosive limits k) Vapour pressureno data available l) Vapour densityno data available m) Relative densityno data available n) Water solubilityinsoluble o) Partition coefficient: n- log Pow: 4,509 octanol/water p) Auto-ignitionno data available temperature q) Decompositionno data available temperature r) Viscosityno data available s) Explosive propertiesno data available t) Oxidizing propertiesno data available Other safety information Solubility in otherChloroform - soluble solventsAcetone - soluble SECTION 10: Stability and reactivity Reactivity no data available Chemical stability Stable under recommended storage conditions. Possibility of hazardous reactions no data available Conditions to avoid no data available Incompatible materials Strong oxidizing agents Hazardous decomposition products Other decomposition products - no data available In the event of fire: see section 5 SECTION 11: Toxicological information Information on toxicological effects Acute toxicity no data available Skin corrosion/irritation no data available Serious eye damage/eye irritation no data available Respiratory or skin sensitisation no data available Germ cell mutagenicity no data available Carcinogenicity IARC:No component of this product present at levels greater than or equal to 0.1% is identified as probable, possible or confirmed human carcinogen by IARC. Reproductive toxicity no data available Specific target organ toxicity - single exposure no data available Specific target organ toxicity - repeated exposure no data available Aspiration hazard no data available Additional Information RTECS: Not available To the best of our knowledge, the chemical, physical, and toxicological properties have not been thoroughly investigated. SECTION 12: Ecological information Toxicity no data available Persistence and degradability no data available Bioaccumulative potential no data available Mobility in soil no data available Results of PBT and vPvB assessment PBT/vPvB assessment not available as chemical safety assessment not required/not conducted Other adverse effects SECTION 13: Disposal considerations Waste treatment methods Product Offer surplus and non-recyclable solutions to a licensed disposal company. Contact a licensed professional waste disposal service to dispose of this material. Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. Contaminated packaging Dispose of as unused product. SECTION 14: Transport information UN number ADR/RID: -IMDG: -IATA: - UN proper shipping name ADR/RID: Not dangerous goods IMDG: Not dangerous goods IATA:Not dangerous goods Transport hazard class(es) ADR/RID: -IMDG: -IATA: - Packaging group ADR/RID: -IMDG: -IATA: - Environmental hazards ADR/RID: noIMDG Marine pollutant: noIATA: no Special precautions for user no data available SECTION 15 - REGULATORY INFORMATION N/A SECTION 16 - ADDITIONAL INFORMATION N/A |
|
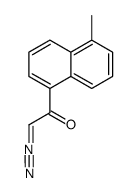
~35% 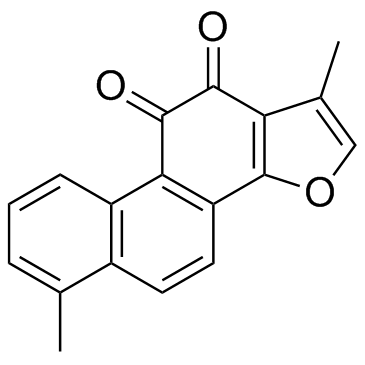
568-73-0 |
| Literature: Jiao, Mingkun; Ding, Chunyong; Zhang, Ao Tetrahedron, 2014 , vol. 70, # 18 p. 2976 - 2981 |
|
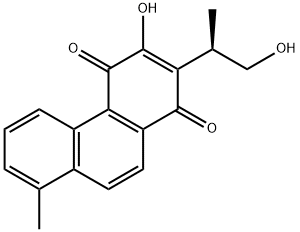
~99% 
568-73-0 |
| Literature: Danheiser, Rick L.; Casebier, David S.; Loebach, Jennifer L. Tetrahedron Letters, 1992 , vol. 33, # 9 p. 1149 - 1152 |
|
~% 
568-73-0 |
| Literature: Tetrahedron Letters, , vol. 33, # 9 p. 1149 - 1152 |
|
~% 
568-73-0 |
| Literature: Tetrahedron Letters, , vol. 33, # 9 p. 1149 - 1152 |
|
~% 
568-73-0 |
| Literature: Tetrahedron Letters, , vol. 33, # 9 p. 1149 - 1152 |
|
~% 
568-73-0 |
| Literature: Tetrahedron Letters, , vol. 33, # 9 p. 1149 - 1152 |
|
~% 
568-73-0 |
| Literature: Tetrahedron, , vol. 70, # 18 p. 2976 - 2981 |
|
~% 
568-73-0 |
| Literature: Tetrahedron, , vol. 70, # 18 p. 2976 - 2981 |
|
~% 
568-73-0 |
| Literature: Tetrahedron, , vol. 70, # 18 p. 2976 - 2981 |
| Precursor 4 | |
|---|---|
| DownStream 0 | |