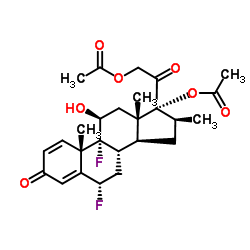
33564-31-7
| Name | diflorasone diacetate |
|---|---|
| Synonyms |
[2-[(6S,8S,9R,10S,11S,13S,14S,16S,17R)-17-acetyloxy-6,9-difluoro-11-hydroxy-10,13,16-trimethyl-3-oxo-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-17-yl]-2-oxoethyl] acetate
(6S,8S,9R,10S,11S,13S,14S,16S,17R)-17-[(acetyloxy)acetyl]-6,9-difluoro-11-hydroxy-10,13,16-trimethyl-3-oxo-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthren-17-yl acetate Diflorasone diacetate (6α,11β,16β)-6,9-Difluoro-11-hydroxy-16-methyl-3,20-dioxopregna-1,4-diene-17,21-diyl diacetate acétate de (6S,8S,9R,10S,11S,13S,14S,16S,17R)-17-[(acétyloxy)acétyl]-6,9-difluoro-11-hydroxy-10,13,16-triméthyl-3-oxo-6,7,8,9,10,11,12,13,14,15,16,17-dodécahydro-3H-cyclopenta[a]phénanthrén-17-yle PSORCON 6a,9-Difluoro-11b,17,21-trihydroxy-16b-methylpregna-1,4-diene-3,20-dione 17,21-Diacetate 6a-Fluorobetamethasone-17,21 Diacetate Diflorasone diacetate [USAN:JAN] MFCD00079159 EINECS 251-575-1 (6S,8S,9R,10S,11S,13S,14S,16S,17R)-17-[(Acetyloxy)acetyl]-6,9-difluor-11-hydroxy-10,13,16-trimethyl-3-oxo-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthren-17-ylacetat Diflorasone diacetate (JAN/USP) |
| Description | Diflorasone diacetate is an anti-inflammatory steroid compound used as locally or topically agent. Diflorasone diacetate is being used for skin disorders to control corticosteroid-responsive dermatoses[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 585.1±50.0 °C at 760 mmHg |
| Melting Point | 47-49 °C(lit.) |
| Molecular Formula | C26H32F2O7 |
| Molecular Weight | 494.525 |
| Flash Point | 307.6±30.1 °C |
| Exact Mass | 494.211609 |
| PSA | 106.97000 |
| LogP | 3.10 |
| Vapour Pressure | 0.0±3.7 mmHg at 25°C |
| Index of Refraction | 1.546 |
| Storage condition | Refrigerator |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |


GHS07, GHS08 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H312 + H332-H351 |
| Precautionary Statements | P261-P280 |
| Hazard Codes | Xn:Harmful; |
| Risk Phrases | R20/21/22;R40 |
| Safety Phrases | S22-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | TU3722000 |
| HS Code | 2942000000 |
| HS Code | 2942000000 |
|---|