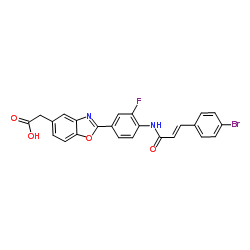
853929-59-6
| Name | 2-[2-[4-[[1-(4-bromophenyl)-3-oxoprop-1-en-2-yl]amino]-3-fluorophenyl]-1,3-benzoxazol-5-yl]acetic acid |
|---|---|
| Synonyms |
W-9 hydrochloride
[2-(4-{[(2E)-3-(4-Bromophenyl)prop-2-enoyl]amino}-3-fluorophenyl)-1,3-benzoxazol-5-yl]acetic acid [2-(4-{[(2E)-3-(4-Bromophenyl)-2-propenoyl]amino}-3-fluorophenyl)-1,3-benzoxazol-5-yl]acetic acid 2-2-4-1-(4-Bromophenyl)-3-oxoprop-1-en-2-ylamino-3-fluorophenyl-1,3-benzoxazol-5-ylacetic acid |
| Description | OGT 2115 is a potent, cell-permeable and orally active heparanase inhibitor with an IC50 of 0.4 μM. OGT 2115 has anti-angiogenic properties (IC50 of 1 μM). OGT 2115 also inhibits heparan sulfate degradation activity[1][2]. |
|---|---|
| Related Catalog | |
| Target |
IC50: 0.4 μM (Heparanase)[1] |
| In Vitro | Heparanase InhibitorOGT 2115 can suppress metastasis induced by endoplasmic reticulum (ER) stress in breast cancer cells, although not significantly. However, compared with the control group, the number and rate of migrated cells are significantly reduced following the exposure of the cells to Tunicamycin + OGT 2115. OGT 2115 significantly inhibits the invasion and migration induced by Adriamycin. Furthermore, the MTT assay results show that OGT 2115 does not decrease the anti-proliferative effect of Adriamycin[2]. |
| In Vivo | When administered to mice, OGT 2115 (Compound 12d) shows a plasma concentration of ~10x the heparanase IC50 following oral dosing at 20 mg/kg[1]. |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 685.9±55.0 °C at 760 mmHg |
| Molecular Formula | C24H16BrFN2O4 |
| Molecular Weight | 495.297 |
| Flash Point | 368.6±31.5 °C |
| Exact Mass | 494.027740 |
| PSA | 92.43000 |
| LogP | 6.30 |
| Vapour Pressure | 0.0±2.2 mmHg at 25°C |
| Index of Refraction | 1.710 |
| Hazard Codes | Xi |
|---|