66-28-4
| Name | strophanthidin |
|---|---|
| Synonyms |
Convallatoxigenin
Corchorgenin Corchsularin Strophanthidin K MFCD00046266 EINECS 200-626-6 (3S,5S,8R,9S,10S,13R,14S,17R)-3,5,14-trihydroxy-13-methyl-17-(5-oxo-2H-furan-3-yl)-2,3,4,6,7,8,9,11,12,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthrene-10-carbaldehyde k-Strophanthidin Strophanthidine Erysimupicrone Corchorin Corchoside A aglycon |
| Description | Strophanthidin is a naturally available cardiac glycoside[1]. Strophanthidin 0.1 and 1 nmol/L increases and 1~100 µmol/L inhibits the Na+/K+-ATPase activities, but Strophanthidin 10 and 100 nmol/L does not affect Na+/K+-ATPase activities in cardiac sarcolemmal[2]. Strophanthidin increases both diastolic and systolic intracellular Ca2+ concentration[3]. |
|---|---|
| Related Catalog | |
| Target |
Na+/K+-ATPase[2] |
| In Vitro | Strophanthidin (0~10 μM; 24 hours; MCF-7, A549, and HepG2 cells) is effective at suppressing the growth of cancer cells and has no toxicity in normal cells[1]. Strophanthidin (0.5 to 500 µM; PBMCs) does not show significant cytotoxicity in PBMCs. Strophanthidin (2 µM; MCF-7 cells) can arrest cell cycle at the G2/M phase[1]. Strophanthidin (MCF-7, A549, and HepG2 cells) is effective at suppressing the growth of cancer cells and has no toxicity in normal cells. Strophanthidin (MCF-7, A549, and HepG2 cells) inhibits the expression of checkpoint and cyclin-dependent kinases in three cancer cells compared to untreated controls. Strophanthidin can modulate the protein localization from the nucleus to the membrane as well as to the cytoplasm. Strophanthidin is a monosaccharide cardiac glycoside with one aglycone portion and without any sugar unit. Strophanthidin induces apoptosis by the attenuation of multiple biochemical signaling pathways and by arresting cell cycle at the G2/M phase through p53-dependent and p53-independent mechanisms[1]. Cell Viability Assay[1] Cell Line: MCF-7, A549, and HepG2 cells Concentration: 0~10 μM Incubation Time: 24 hours Result: Inhibited the proliferation in three different cancer cells. |
| References |
| Density | 1.432 g/cm3 |
|---|---|
| Boiling Point | 620.7ºC at 760 mmHg |
| Melting Point | 169ºC |
| Molecular Formula | C23H32O6 |
| Molecular Weight | 404.49700 |
| Flash Point | 214.9ºC |
| Exact Mass | 404.22000 |
| PSA | 104.06000 |
| LogP | 1.89820 |
| Index of Refraction | 1.674 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H300-H310-H330 |
| Precautionary Statements | P260-P264-P280-P284-P302 + P350-P310 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | T+: Very toxic; |
| Risk Phrases | R26/27/28 |
| Safety Phrases | S22;S36/S37/S39;S45 |
| RIDADR | UN 2811 6 |
| RTECS | FH5425000 |
|
~82% 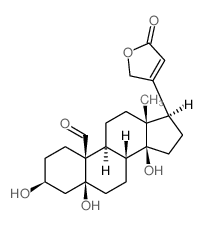
66-28-4 |
| Literature: Makarevich, I. F.; Tishchenko, A. A.; Terno, I. S. Chemistry of Natural Compounds, 1991 , vol. 27, # 1 p. 53 - 55 Khimiya Prirodnykh Soedinenii, 1991 , # 1 p. 62 - 64 |
|
~%
Detail
|
| Literature: Chemistry of Natural Compounds, , vol. 22, # 2 p. 186 - 189 Khimiya Prirodnykh Soedinenii, , vol. 22, # 2 p. 201 - 204 |
|
~% 
66-28-4 |
| Literature: Gazzetta Chimica Italiana, , vol. 112, # 9/10 p. 349 - 352 |
| Precursor 3 | |
|---|---|
| DownStream 4 | |
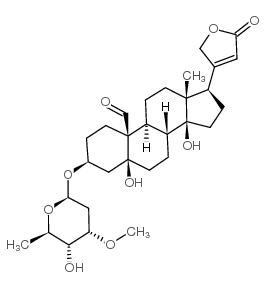
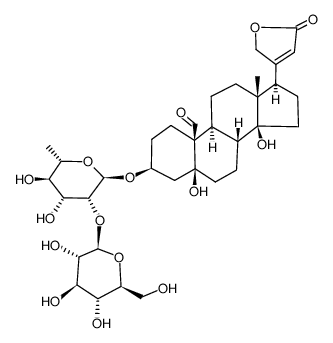



![(3S,5S,10S,13R,14S,17R)-5,14-dihydroxy-13-methyl-17-(5-oxo-2H-furan-3-yl)-3-[(2S,5R)-3,4,5-trihydroxyoxan-2-yl]oxy-2,3,4,6,7,8,9,11,12,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthrene-10-carbaldehyde structure](https://image.chemsrc.com/caspic/116/15596-26-6.png)
![[10-formyl-5,14-dihydroxy-13-methyl-17-(5-oxo-2H-furan-3-yl)-2,3,4,6,7,8,9,11,12,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-yl] 2-iodoacetate structure](https://image.chemsrc.com/caspic/426/4956-17-6.png)
