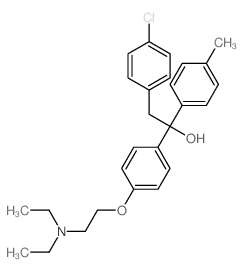
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
KK2400000
-
CHEMICAL NAME :
-
Ethanol, 2-(p-chlorophenyl)-1-(p-(2-(diethylamino)ethoxy)pheny l)-1-p-tolyl-
-
CAS REGISTRY NUMBER :
-
78-41-1
-
LAST UPDATED :
-
199612
-
DATA ITEMS CITED :
-
13
-
MOLECULAR FORMULA :
-
C27-H32-Cl-N-O2
-
MOLECULAR WEIGHT :
-
438.05
-
WISWESSER LINE NOTATION :
-
GR D1XQR D1&R DO2N2&2
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
2 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
CRSBAW Comptes Rendus des Seances de la Societe de Biologie et de Ses Filiales. (SPPIF, B.P.22, F-41353 Vineuil, France) V.1- 1849- Volume(issue)/page/year: 155,2255,1961 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
14 mg/kg
-
SEX/DURATION :
-
female 1-14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
TXAPA9 Toxicology and Applied Pharmacology. (Academic Press, Inc., 1 E. First St., Duluth, MN 55802) V.1- 1959- Volume(issue)/page/year: 10,565,1967
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
450 mg/kg
-
SEX/DURATION :
-
female 6-14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Specific Developmental Abnormalities - homeostasis Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue)
-
REFERENCE :
-
TXAPA9 Toxicology and Applied Pharmacology. (Academic Press, Inc., 1 E. First St., Duluth, MN 55802) V.1- 1959- Volume(issue)/page/year: 10,565,1967
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
350 mg/kg
-
SEX/DURATION :
-
female 2-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - eye/ear Reproductive - Specific Developmental Abnormalities - urogenital system Reproductive - Specific Developmental Abnormalities - homeostasis
-
REFERENCE :
-
CRSBAW Comptes Rendus des Seances de la Societe de Biologie et de Ses Filiales. (SPPIF, B.P.22, F-41353 Vineuil, France) V.1- 1849- Volume(issue)/page/year: 167,1523,1973
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
350 mg/kg
-
SEX/DURATION :
-
female 2-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - abortion Reproductive - Effects on Embryo or Fetus - fetal death Reproductive - Specific Developmental Abnormalities - Central Nervous System
-
REFERENCE :
-
CRSBAW Comptes Rendus des Seances de la Societe de Biologie et de Ses Filiales. (SPPIF, B.P.22, F-41353 Vineuil, France) V.1- 1849- Volume(issue)/page/year: 167,1523,1973
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
70 mg/kg
-
SEX/DURATION :
-
female 14 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - parturition Reproductive - Effects on Newborn - stillbirth Reproductive - Effects on Newborn - live birth index (measured after birth)
-
REFERENCE :
-
ENDOAO Endocrinology (Baltimore). (Williams & Wilkins Co., 428 E. Preston St., Baltimore, MD 21203) V.1- 1917- Volume(issue)/page/year: 74,64,1964
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
70 mg/kg
-
SEX/DURATION :
-
female 14 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - eye/ear
-
REFERENCE :
-
ENDOAO Endocrinology (Baltimore). (Williams & Wilkins Co., 428 E. Preston St., Baltimore, MD 21203) V.1- 1917- Volume(issue)/page/year: 74,64,1964
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
2800 mg/kg
-
SEX/DURATION :
-
female 1-14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - eye/ear Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue) Reproductive - Specific Developmental Abnormalities - urogenital system
-
REFERENCE :
-
CRSBAW Comptes Rendus des Seances de la Societe de Biologie et de Ses Filiales. (SPPIF, B.P.22, F-41353 Vineuil, France) V.1- 1849- Volume(issue)/page/year: 160,923,1966
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
2800 mg/kg
-
SEX/DURATION :
-
female 1-14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetal death
-
REFERENCE :
-
CRSBAW Comptes Rendus des Seances de la Societe de Biologie et de Ses Filiales. (SPPIF, B.P.22, F-41353 Vineuil, France) V.1- 1849- Volume(issue)/page/year: 160,923,1966 *** REVIEWS *** TOXICOLOGY REVIEW CRTXB2 CRC Critical Reviews in Toxicology. (CRC Press, Inc., 2000 Corporate Blvd., NW, Boca Raton, FL 33431) V.1- 1971- Volume(issue)/page/year: 1(1),93,1971 TOXICOLOGY REVIEW CLPTAT Clinical Pharmacology and Therapeutics (St. Louis). (C.V. Mosby Co., 11830 Westline Industrial Dr., St. Louis, MO 63146) V.1- 1960- Volume(issue)/page/year: 5,480,1964 TOXICOLOGY REVIEW ARVPAX Annual Review of Pharmacology. (Palo Alto, CA) V.1-15, 1961-75. For publisher information, see ARPTDI. Volume(issue)/page/year: 5,447,1965 TOXICOLOGY REVIEW ADVPA3 Advances in Pharmacology. (New York, NY) V.1-6, 1962-68. For publisher information, see AVPCAQ. Volume(issue)/page/year: 4,263,1966
|