158563-45-2
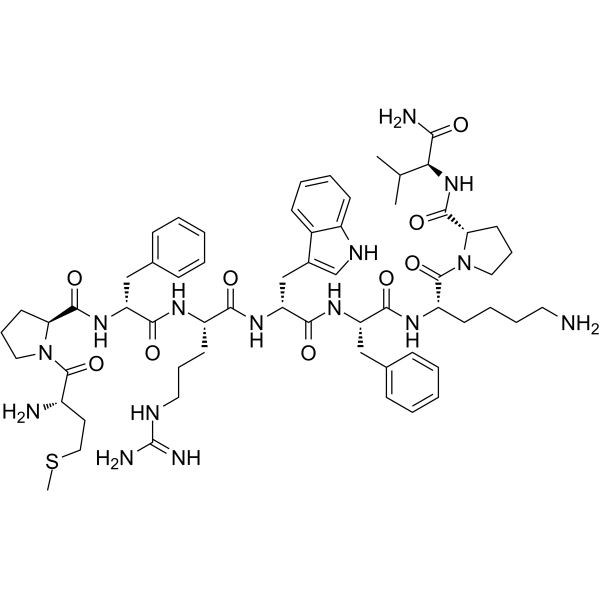
| Name | (2S)-1-[(2S)-6-amino-2-[[(2S)-2-[[(2R)-2-[[(2S)-2-[[(2R)-2-[[(2S)-1-[(2S)-2-amino-4-methylsulfanyl-butanoyl]pyrrolidine-2-carbonyl]amino]-3-phenyl-propanoyl]amino]-5-guanidino-pentanoyl]amino]-3-(1H-indol-3-yl)propanoyl]amino]-3-phenyl-propanoyl]amino]hexanoyl]-N-[(1S)-1-carbamoyl-2-methyl-propyl]pyrrolidine-2-carboxamide |
|---|---|
| Synonyms |
(S)-1-(L-methionyl)-N-((R)-1-(((S)-1-(((R)-1-(((S)-1-(((S)-6-amino-1-((S)-2-(((S)-1-amino-3-methyl-1-oxobutan-2-yl)carbamoyl)pyrrolidin-1-yl)-1-oxohexan-2-yl)amino)-1-oxo-3-phenylpropan-2-yl)amino)-3-(1H-indol-3-yl)-1-oxopropan-2-yl)amino)-5-guanidino-1-oxopentan-2-yl)amino)-1-oxo-3-phenylpropan-2-yl)pyrrolidine-2-carboxamide
Nonapeptide-1 Melitane H-Met-Pro-D-Phe-Arg-D-Trp-Phe-Lys-Pro-Val-NH2 L-Methionyl-L-prolyl-D-phenylalanyl-L-arginyl-D-tryptophyl-L-phenylalanyl-L-lysyl-L-prolyl-L-valinamide Melanostatine |
| Description | Nonapeptide-1 (Melanostatine-5), a peptide hormone, is a selective antagonist of MC1R (Ki: 40 nM). Nonapeptide-1 is a competitive α-MSH antagonist that potently inhibits intracellular cAMP and melanosome dispersion induced by α-MSH in melanocytes (IC50: 2.5 nM and 11 nM, respectively). Nonapeptide-1 inhibits melanin synthesis, and can be used in the research of skin pigmentation and regulation of steroid production in the adrenal gland, skin cancer[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
MC1R:40 nM (Ki) MC3R:0.47 μM (Ki) MC4R:1.34 μM (Ki) MC5R:2.4 μM (Ki) |
| In Vitro | Nonapeptide-1 (153N-6) inhibits α-melanocyte hormone (α-MSH)-induced melanosome dispersion, with an IC50 value of 11 nM[1]. Nonapeptide-1 (0.1 nM-1 μΜ, 30 min) inhibits α-MSH-induced intracellular cAMP levels in melanocytes, with an IC50 of 2.5 nM[1]. Nonapeptide-1 (153N-6) shows highest affinity for MC1R (Ki: 40 nM) in COS-1 cells expressing human receptors, and is selective for MC1R over MC3R, MC4R, and MC5R (Ki: 0.47, 1.34, and 2.4 μΜ, respectively)[2]. Nonapeptide-1 (N-1A, 20 μΜ, 3 days) inhibits the basal melanin synthesis and reverses UVA-induced melanin increase in Human epidermal melanocytes (HEM cells) and HaCaT cells[3]. Nonapeptide-1 (20 μΜ, 3 days) competes with α-MSH and downregulates the expression of MC1R, tyrosinase, TRP1, TRP2, and MITF via binding to MC1R in HaCaT cells and HEM cells[3]. Western Blot Analysis[3] Cell Line: HaCaT cells, Human epidermal melanocytes (HEM) Concentration: 20 μΜ Incubation Time: 3 days Result: Downregulated the expression of MC1R, tyrosinase, TRP1, TRP2, and MITF. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Molecular Formula | C61H87N15O9S |
| Molecular Weight | 1206.504 |
| Exact Mass | 1205.653198 |
| PSA | 413.34000 |
| LogP | 3.90 |
| Index of Refraction | 1.672 |