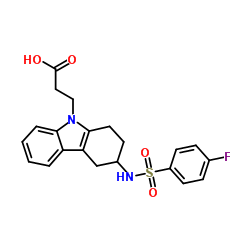
627865-18-3
| Name | 2-[3-[(4-fluorophenyl)sulfonylmethylamino]-1,2,3,4-tetrahydrocarbazol-9-yl]acetic acid |
|---|---|
| Synonyms | 3-(3-{[(4-Fluorophenyl)sulfonyl]amino}-1,2,3,4-tetrahydro-9H-carbazol-9-yl)propanoic acid |
| Description | CAY10471 (TM30089) is a potent, selective, and orally active prostaglandin D2 receptor CRTH2 antagonist. CAY10471 attenuates the progression of tubulointerstitial fibrosis and chronic contact hypersensitivity (CHS) in animal model[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
hCRTH2 |
| In Vitro | CAY10471 (1 μM; 1-24 hours) decreases 15dPGJ2-induced phosphorylation of p38 MAP kinase significantly in PC12 cells[1]. Western Blot Analysis[1] Cell Line: PC12 cells Concentration: 1 μM Incubation Time: 1 or 24 hours Result: Blocked 15d-PGJ2-induced p38 MAP kinase activation. |
| In Vivo | CAY10471 (oral treatment; 2 mg/kg; challenged on day 22 or over 10 consecutive days) shows a diminished inflammation in chronic contact hypersensitivity (CHS) and IgE-CAI model. It blocks CRTH2 partly, but significantly suppresses inflammation in mice[2]. CAY10471 (oral adminstration; 20 mg/kg; twice daily; beginning 3/4/5 days before UUO) significantly attenuates interstitial collagen deposition in the cortex when compared with the vehicle (8.40% versus 14.85%). Oral administration from 3 days after UUO also significantly attenuates interstitial collagen deposition in the cortex compared with vehicle (9.63% versus 14.44%). However, oral administration beginning 5 days after UUO has little effect on interstitial collagen deposition in the cortex when compared with vehicle (14.61% versus 15.09%). Unilateral ureteral obstruction (UUO)[3]. Animal Model: Balb/c mice, DP−/− mice, CRTH2−/− mice[2] Dosage: 2 mg/kg Administration: Oral treatment; once daily; challenged on day 22 or over 10 consecutive days Result: Significantly suppressed both CHS and IgE-CAI inflammatory responses. Animal Model: C57BL/6 mice[3] Dosage: 20 mg/kg Administration: Oral treatment; twice daily; beginning 3/4/5 days before UUO Result: Slowed the progression of renal fibrosis in the obstructed kidneys. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 654.7±65.0 °C at 760 mmHg |
| Molecular Formula | C21H21FN2O4S |
| Molecular Weight | 416.466 |
| Flash Point | 349.7±34.3 °C |
| Exact Mass | 416.120605 |
| PSA | 87.99000 |
| LogP | 4.09 |
| Vapour Pressure | 0.0±2.1 mmHg at 25°C |
| Index of Refraction | 1.664 |