19774-82-4
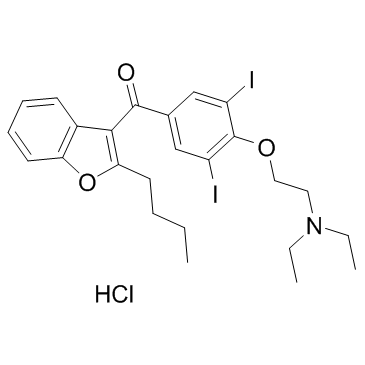
| Name | Amiodarone Hydrochloride |
|---|---|
| Synonyms |
(2-Butyl-1-benzofuran-3-yl){4-[2-(diethylamino)ethoxy]-3,5-diiodophenyl}methanone hydrochloride
(2-Butyl-3-benzofuranyl)[4-[2-(diethylamino)e MFCD08064189 (2-Butyl-1-benzofur-3-yl){4-[2-(diethylamino)ethoxy]-3,5-diiodphenyl}methanonhydrochlorid (2-Butyl-1-benzofuran-3-yl){4-[2-(diethylamino)ethoxy]-3,5-diiodophenyl}methanone hydrochloride (1:1) Ancaron (2-butylbenzo[b]furan-3-yl){4-[2-(diethylamino)ethoxy]-3,5-diiodophenyl}methanone hydrochloride Cordarone (2-Butylbenzofuran-3-yl)(4-(2-(diethylamino)ethoxy)-3,5-diiodophenyl)methanone hydrochloride (2-butyl-1-benzofuran-3-yl)-[4-[2-(diethylamino)ethoxy]-3,5-diiodophenyl]methanone,hydrochloride EINECS 243-293-2 methanone, (2-butyl-3-benzofuranyl)[4-[2-(diethylamino)ethoxy]-3,5-diiodophenyl]-, hydrochloride Amiodarone HCl Methanone, (2-butyl-3-benzofuranyl)[4-[2-(diethylamino)ethoxy]-3,5-diiodophenyl]-, hydrochloride (1:1) UNII-976728SY6Z (2-butyl-1-benzofur-3-yl){4-[2-(diéthylamino)éthoxy]-3,5-diiodophényl}méthanone chlorhydrate Ketone, 2-butyl-3-benzofuranyl 4-[2-(diethylamino)ethoxy]-3,5-diiodophenyl, hydrochloride PACERONE Amiodarone hydrochloride Amiodarone (hydrochloride) |
| Description | Amiodarone is an antiarrhythmic drug for inhibition of ATP-sensitive potassium channel with IC50 of 19.1 μM. IC50 Value: 1.5 uM ( inhibit TBARS, LOOH and FPL formation)[1]in vitro: It was found that 10 uM amiodarone induces accumulation of ethidium bromide (5 ug/ml) in Saccharomyces cerevisiae cells. At the same time, in yeast cells with inactivated MDR genes, accumulation of ethidium bromide was 6-fold higher even without amiodarone. Addition of non-lethal concentrations of amiodarone to MDR-deficient cells caused an increase of intracellular ethidium bromide to the level, which was even lower than the level in amiodarone-treated wild-type cells [2]. Cells treated with amiodarone were seen to have detached from the dish, with cell rounding, cytoplasmic blebbing and irregularity in shape. An increase in the sub-G1 phase fraction, from 15.43 to 21.34% and 79.83% and a reduction in the G1 phase fraction, from 48.83 to 41.63% and 11.52%, were observed in cells treated with amiodarone at concentrations of 0.1 and 1 mM, respectively [3].in vivo: Chronic treatment with oral amiodarone for 4 weeks reduced i.p. when myocytes were dialyzed with patch-pipettes containing either 10 mM Na+ or 80 mM Na+. In myocytes from untreated rabbits, acute exposure to amiodarone in vitro reduced i.p. when patch pipettes contained 10 mM Na+ but had no effect on i.p. at 80 mM Na+. Amiodarone had no effect on the voltage dependence of the pump or the affinity of the pump for extracellular K+ either after chronic treatment or during acute exposure [4].Clinical trial: Continuous Versus Episodic Amiodarone Treatment for the Prevention of Permanent Atrial Fibrillation . Phase not specified |
|---|---|
| Related Catalog | |
| References |
| Density | 1.58 g/cm3 |
|---|---|
| Boiling Point | 635.1ºC at 760 mmHg |
| Melting Point | 154-158°C |
| Molecular Formula | C25H30ClI2NO3 |
| Molecular Weight | 681.773 |
| Flash Point | 337.9ºC |
| Exact Mass | 681.000366 |
| PSA | 42.68000 |
| LogP | 7.73820 |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |



GHS02, GHS06, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H225-H301 + H311 + H331-H370 |
| Precautionary Statements | P210-P260-P280-P301 + P310-P311 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R20/21/22 |
| Safety Phrases | S36 |
| RIDADR | 1230.0 |
| WGK Germany | 3 |
| RTECS | OB1361000 |
| Hazard Class | 3、6.1 |
| HS Code | 2932999099 |
| HS Code | 2932999099 |
|---|---|
| Summary | 2932999099. other heterocyclic compounds with oxygen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |