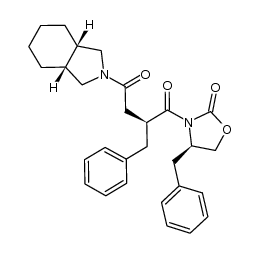
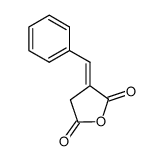
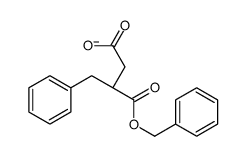
145375-43-5
| Name | [2(S)-cis]-Octahydro-γ-oxo-α-(phenylmethyl)-2H-isoindole-2-butanoic acid |
|---|---|
| Synonyms |
Mitiglinide
Unii-D86I0xlb13 (-)-(2S,3a,7a-cis)-α-Benzylhexahydro-γ-oxo-2-isoindolinebutyric acid (2S)-2-Benzyl-4-((3a,7a-cis)-octahydro-2H-isoindol-2-yl)-4-oxobutanoic Acid (-)-(2S,3a,7a-cis)-a-Benzylhexahydro-g-oxo-2-isoindolinebutyric Acid (2S)-2-Benzyl-4-[(3aR,7aS)-octahydro-2H-isoindol-2-yl]-4-oxobutanoic acid |
| Description | Mitiglinide (KAD-1229), an insulinotropic agent, is an ATP-sensitive K+ (KATP) channel antagonist. Mitiglinide is highly specific to the Kir6.2/SUR1 complex (the pancreatic beta-cell KATP channel). Mitiglinide can be used for the research of type 2 diabetes[1][2]. |
|---|---|
| Related Catalog | |
| Target |
KATP channel[1] |
| In Vitro | Mitiglinide inhibits the Kir6.2/SUR1 channel currents in a dose-dependent manner (IC50 of 100 nM) but does not significantly inhibit either Kir6.2/SUR2A or Kir6.2/SUR2B channel currents even at high doses (more than 10 μM) in COS-1 cells[1]. |
| In Vivo | Mitiglinide (1-3 mg/kg; p.o.) suppresses the increase in plasma glucose levels seen after a meal load and the area under the curve for plasma glucose levels (AUCglucose) up to 5 h after the meal load[2]. Animal Model: Pregnant Wistar rats (12 weeks)[2] Dosage: 0.3 mg/kg, 1 mg/kg, 3 mg/kg Administration: Oral administration Result: Dose-dependently suppressed AUCglucose levels. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 519.6±43.0 °C at 760 mmHg |
| Molecular Formula | C19H25NO3 |
| Molecular Weight | 315.41 |
| Flash Point | 268.0±28.2 °C |
| Exact Mass | 315.183441 |
| PSA | 57.61000 |
| LogP | 4.73 |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.567 |
| HS Code | 2942000000 |
|---|
| Precursor 5 | |
|---|---|
| DownStream 0 | |
| HS Code | 2942000000 |
|---|
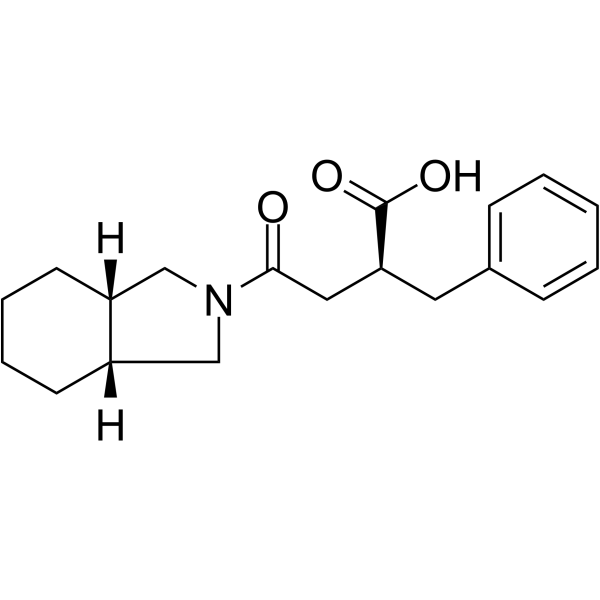
![(3aS,6R,7aS)-8,8-dimethyl-1-{(2S)-4-[(3aR,7aS)-octahydro-2H-isoindol-2-yl]-4-oxo-2-(phenylmethyl)butanoyl}hexahydro-3a,6-methano-2,1-benzisothiazole 2,2-dioxide structure](https://image.chemsrc.com/caspic/489/787635-24-9.png)