7481-89-2
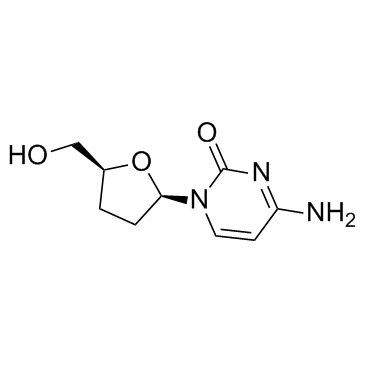
| Name | zalcitabine |
|---|---|
| Synonyms |
ddCyd
[3H]-Zalcitabine 2',3'-DIDEOXYCYTIDINE 4-Amino-1-[(2R,5S)-5-(hydroxymethyl)tetrahydrofuran-2-yl]pyrimidin-2(1H)-one DDC DDC (VAN) Zalcitabine 1-[(2R,5S)-5-(Hydroxymethyl)tetrahydro-2-furanyl]-4-imino-1,4-dihydro-2-pyrimidinol 4-amino-1-[(2R,5S)-5-(hydroxymethyl)oxolan-2-yl]pyrimidin-2-one HIVID MFCD00012188 4-Amino-1-[(2R,5S)-5-(hydroxymethyl)tetrahydro-2-furanyl]-2(1H)-pyrimidinone Cytidine,2',3'-dideoxy Dideoxycytidine 3'-Azido-3'-deoxythymidine 4-Amino-1-[(2R,5S)-5-(hydroxymethyl)tetrahydrofuran-2-yl]pyrimidin-2(1H)-on Zalcitibine 2',3'-Dideoxycytidinene ddC (Antiviral) 4-amino-1-[(2R,5S)-5-(hydroxyméthyl)tétrahydrofuran-2-yl]pyrimidin-2(1H)-one |
| Description | Zalcitabine is a potent nucleoside analogue reverse transcriptase inhibitor used in the treatment of HIV infection. |
|---|---|
| Related Catalog | |
| Target |
Target: HIV |
| In Vitro | Zalcitabine is a dideoxynucleoside antiretroviral agent that is phosphorylated to the active metabolite 2',3'-dideoxycytidine 5'-triphosphate (ddCTP) within both uninfected and HIV-infected cells. At therapeutic concentrations, ddCTP inhibits HIV replication by inhibiting the enzyme reverse transcriptase and terminating elongation of the proviral DNA chain[1]. Zalcitabine exhibits the inhibition effect on the cellular uptake of [3H]-PAH in CHO/hOAT1 cells with an IC50 value of 1.23 mM. Furthermore, the cellular uptake of zalcitabine increased threefold with the enhancement of hOATI activity in CHO/hOAT1 cells[2]. |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 415.0±55.0 °C at 760 mmHg |
| Melting Point | 217-218 °C(lit.) |
| Molecular Formula | C9H13N3O3 |
| Molecular Weight | 211.218 |
| Flash Point | 204.8±31.5 °C |
| Exact Mass | 211.095688 |
| PSA | 90.37000 |
| LogP | -1.30 |
| Vapour Pressure | 0.0±2.2 mmHg at 25°C |
| Index of Refraction | 1.686 |
| Storage condition | 2-8°C |
| Stability | Stable. Combustible. Incompatible with strong oxidizing agents. |
| Water Solubility | 5-10 g/100 mL at 19 ºC |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS08 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H351 |
| Precautionary Statements | P280 |
| Personal Protective Equipment | Eyeshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R40 |
| Safety Phrases | S22-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | HA3870000 |
| HS Code | 2934999090 |
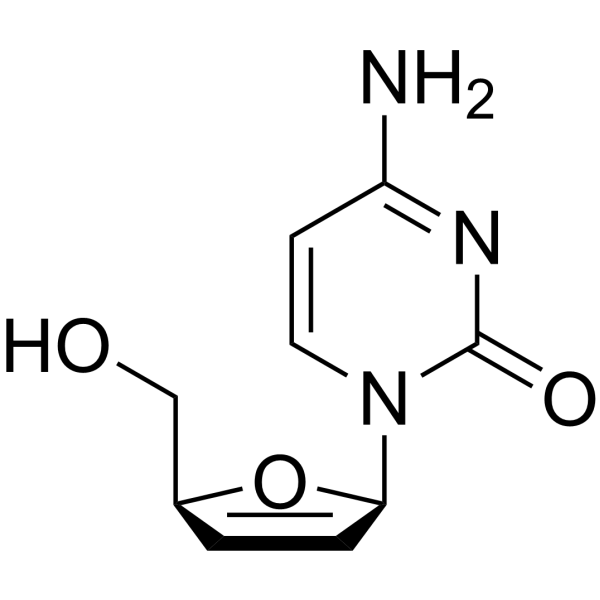
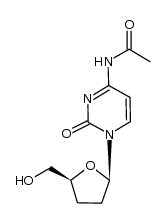
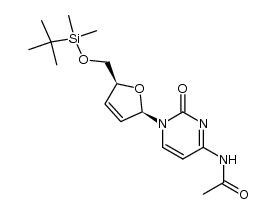
| Precursor 7 | |
|---|---|
| DownStream 1 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |


![1-[2,3-dideoxy-5-O-(4-phenybenzoyl)-β-D-glycero-pentofuranosyl]-4-(isobutyrylamino)-2(1H)-pyrimidinone*0.25H2O structure](https://image.chemsrc.com/caspic/463/125612-00-2.png)

![N-[1-[(2R,5S)-5-(hydroxymethyl)oxolan-2-yl]-2-oxopyrimidin-4-yl]benzamide structure](https://image.chemsrc.com/caspic/433/120885-60-1.png)
