22255-22-7
| Name | 1,3-dimethoxy-5-[(E)-2-(4-methoxyphenyl)ethenyl]benzene |
|---|---|
| Synonyms |
Trimethoxy-trans-stilbene
3,4',5-Trimethoxy-trans-stilbene trimethoxy-trans-resveratrol 1,3-dimethoxy-5-[(E)-2-(4-methoxyphenyl)ethenyl]benzene TRISMETHOXYRESVERATROL resverarol trimethyl ether 1,3-Dimethoxy-5-[(E)-2-(4-methoxyphenyl)vinyl]benzene 1,3-dimethoxy-5-[(1E)-2-(4-methoxyphenyl)ethenyl]-benzene methylated trans-resveratrol (E)-1,3-Dimethoxy-5-(4-methoxystyryl)benzene 3,5,4'-Trimethoxystilbene E-Resveratrol Trimethyl Ether Resveratrol trimethyl ether 3,4',5-trimethoxystilbene Tri-O-methylresveratrol 3,5,4'-TRIMETHOXY-TRANS-STILBENE 1,3-Dimethoxy-5-[(E)-2-(4-methoxy-phenyl)-vinyl]-benzene O-permethylated E-resveratrol trans-Trimethoxyresveratrol |
| Description | Trans-Trimethoxyresveratrol is a derivative of Resveratrol (RSV),and it may be a more potent anti-inflammatory, antiangiogenic and vascular-disrupting agent when compared with resveratrol.In vitro: The in vitro study of resveratrol and trans-Trimethoxyresveratrol showed rather weak cytotoxic effects on three cancer cell lines (HepG2, MCF-7, and MDA-MB-231), which contradicted a previous study reporting that resveratrol inhibited MCF-7 cells with an IC50 of about 10?μM. This discrepancy might be explained by the fact that the measurements were made 24?h after drug treatment, whereas the measurements of the previous study were taken 6 days after. The fact that the cytotoxic effect of trans-Trimethoxyresveratrol was lower than that of resveratrol is surprising, because in many studies, trans-Trimethoxyresveratrol is the most active analogue of resveratrol , although resveratrol shows much stronger antioxidant effects than that of trans-Trimethoxyresveratrol.[1]In vivo: Zebrafish embryos offer great advantage over their adults as well as other in vivo models because of the external development and optical transparency during their first few days, making them invaluable in the inspection of developmental processes. These unique advantages can even be made more useful when specific cell types are labeled with fluorescent probes. Zebrafish embryo in vivo, suggests that trans-Trimethoxyresveratrol has both more potent antiangiogenic activity and more importantly, stronger specific cytotoxic effects on endothelial cells than does resveratrol.[1] |
|---|---|
| Related Catalog | |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 423.8±35.0 °C at 760 mmHg |
| Melting Point | 57ºC |
| Molecular Formula | C17H18O3 |
| Molecular Weight | 270.323 |
| Flash Point | 144.4±23.2 °C |
| Exact Mass | 270.125580 |
| PSA | 27.69000 |
| LogP | 4.61 |
| Vapour Pressure | 0.0±1.0 mmHg at 25°C |
| Index of Refraction | 1.600 |
| Storage condition | ?20°C |
| Water Solubility | DMSO: ≥34mg/mL |
| Symbol |



GHS05, GHS07, GHS09 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H315-H318-H335-H410 |
| Precautionary Statements | P261-P273-P280-P305 + P351 + P338-P501 |
| Hazard Codes | Xi,N |
| Risk Phrases | 36/37/38 |
| Safety Phrases | 26-36/37/39 |
| RIDADR | UN 3077 9 / PGIII |
| HS Code | 2909309090 |
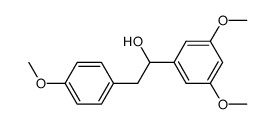
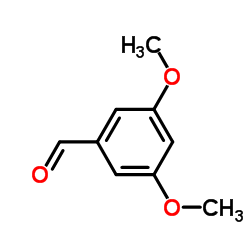
| Precursor 10 | |
|---|---|
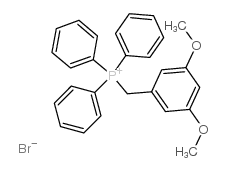
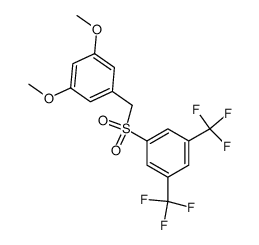
| DownStream 2 | |
| HS Code | 2909309090 |
|---|---|
| Summary | 2909309090 other aromatic ethers and their halogenated, sulphonated, nitrated or nitrosated derivatives VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:5.5% General tariff:30.0% |


![[3,5-bis(trifluoromethyl)phenyl] 3,5-dimethoxybenzyl sulfide structure](https://image.chemsrc.com/caspic/148/863560-56-9.png)