21200-24-8
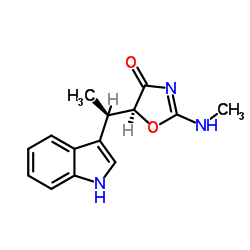
| Name | indolmycin |
|---|---|
| Synonyms |
[S-(R*,S*)]-5-[1-(1H-Indol-3-yl)ethyl]-2-(methylamino)-4(5H)-oxazolone
(1R,5S)-(-)-5-(1-Indol-3-ylethyl)-2-(methylamino)-2-oxazolin-4-one (5S)-5-[(1R)-1-(1H-Indol-3-yl)ethyl]-2-(methylamino)-1,3-oxazol-4(5H)-one Indolemycin 2-Methylamino-5a-(b-indolyl)ethyl-2-oxazolin-4-one Pa 155A indolmycin 2-Oxazolin-4-one,5-(1-indol-3-ylethyl)-2-(methylamino)-,(1R,5S)-(-) 4(5H)-Oxazolone,5-(1-(1H-indol-3-yl)ethyl)-2-(methylamino)-,(S-(R*,S*)) PA 155 A 5-(1-Indol-3-ylethyl)-2-(methylamino)-2-oxazolin-4-one Indomycin (5S)-5-[(1R)-1-(1H-indol-3-yl)ethyl]-2-(methylamino)-1,3-oxazol-4-one |
| Description | Indolmycin (TAK-083), an antibiotic, is a competitive inhibitor of prokaryotic tryptophanyl-tRNA ligase (TrpS). Indolmycin (TAK-083) possesses both anti-viral and anti-bacterial activity[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | Indolmycin was bacteriostatic and demonstrated good activity against MSSA (methicillinsusceptible Staphylococcus aureus), MRSA (methicillin-resistant S. aureus) and VISA (vancomycinintermediate S. aureus), including strains resistant to mupirocin or fusidic acid. Indolmycin MICs for 20 strains ranged from 8 to 32 mg/L, whereas asingle strain exhibited high-level resistance (MIC 128 mg/L)[2]. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 420.8±37.0 °C at 760 mmHg |
| Molecular Formula | C14H15N3O2 |
| Molecular Weight | 257.288 |
| Flash Point | 208.3±26.5 °C |
| Exact Mass | 257.116425 |
| PSA | 69.97000 |
| LogP | 1.53 |
| Vapour Pressure | 0.0±1.0 mmHg at 25°C |
| Index of Refraction | 1.670 |
| HS Code | 2934999090 |
|---|
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |