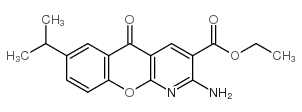
68302-57-8
| Name | 2-amino-5-oxo-7-propan-2-ylchromeno[2,3-b]pyridine-3-carboxylic acid |
|---|---|
| Synonyms |
2-amino-7-(1-methylethyl)-5-oxo-5H-chromeno[2,3-b]pyridine-3-carboxylic acid
Amlexanoxum 2-Amino-7-isopropyl-5-oxo-5H-[1]benzopyrano[2,3-b]pyridine-3-carboxylic Acid Amlenanox Amlexanox Amlexanoxo Elics Amoxanox Solfa MFCD00864790 2-Amino-7-(1-methylethyl)-5-oxo-5H-[1]benzopyrano[2,3-b]pyridine-3-carboxylicAcid amlexanoxum [INN_la] Aphthasol 2-Amino-7-isopropyl-1-azaxanthone-3-carboxylic Acid [14C]-Amlexanox Amlexanoxum [Latin] 2-Amino-7-isopropyl-5-oxo-5H-chromeno[2,3-b]pyridine-3-carboxylic acid 2-amino-5-oxo-7-(propan-2-yl)-5H-chromeno[2,3-b]pyridine-3-carboxylic acid |
| Description | AmLexanox is a specific inhibitor of IKKε and TBK1, and inhibits the IKKε and TBK1 activity determined by MBP phosphorylation with an IC50 of approximately 1-2 μM. |
|---|---|
| Related Catalog | |
| Target |
IKKε:1-2 μM (IC50) TBK1:1-2 μM (IC50) |
| In Vitro | AmLexanox increases phosphorylation of TBK1 on Ser172 in 3T3-L1 adipocytes, and blocks polyinosinic:polycytidylic acid (poly I:C)-stimulated phosphorylation of interferon responsive factor-3 (IRF3), a presumed substrate of IKKε and TBK1[1]. AmLexanox potently inhibits the release of histamine and leukotrienes from mast cells, basophils and neutrophils in in vitro settings, possibly through increasing intracellular cyclic AMP content in inflammatory cells, a mem-brane-stabilising effect or inhibition of calcium influx[2]. In primary bone marrow derived macrophages (BMMs), amLexanox inhibits osteoclast formation and bone resorption. At the molecular level, amLexanox suppresses RANKL-induced activation of nuclear factor-κB (NF-κB), mitogen-activated protein kinase (MAPKs), c-Fos and NFATc1. AmLexanox decreases the expression of osteoclast-specific genes, including TRAP, MMP9, Cathepsin K and NFATc1[3]. |
| In Vivo | AmLexanox (100 mg/kg, p.o.) prevents and reverses diet-induced or genetic obesity, and produces reversible weight loss in obese mice. AmLexanox also causes a significant decrease in adipose tissue mass in these mice, and an increase in circulating adiponectin. AmLexanox (25 mg/kg) significantly improves insulin sensitivity in mice with established DIO,and after four weeks of treatment, amLexanox produces marked improvements in glucose[1]. AmLexanox before the first application of the paste and at each has been shown to suppress both immediate and evaluation thereafter. A categorical scale is also delayed-type hypersensitivity reactions[2]. AmLexanox (20 mg/kg) enhances osteoblast differentiation of BMSCs. In ovariectomized (OVX) mouse model, amLexanox prevents OVX-induced bone loss by suppressing osteoclast activity[3]. |
| Kinase Assay | The in vitro kinase assays is performed by incubating purified kinase (IKKε or TBK1) in kinase buffer containing 25 mM Tris (pH7.5), 10 mM MgCl2, 1 mM DTT, and 10 µM ATP for 30 minutes at 30°C in the presence of 0.5 µCi γ-[32P]-ATP and 1 µg MBP per sample as a substrate. The kinase reaction is stopped by adding 4x sodium dodecyl sulfate (SDS) sample buffer and boiling for 5 minutes at 95°C. Supernatants are resolved by SDS-polyacrylamide gel electrophoresis, transferred to nitrocellulose, and analyzed by autoradiography using a Typhoon 9410 phosphorimager. |
| Cell Assay | To examine cell proliferation, a Cell Counting Kit-8 is used according to the manufacturer’s instructions. BMMs are seeded at a density of 5×103 cells/well in 96-well plates. After 24 hours, cells are treated with different concentrations of AmLexanox (0, 1.5, 3, 6, 12, 25 μM) every 2 days in the presence of M-CSF (30 ng/mL) for 7 days. After 1, 3, 5 and 7 days, the culture medium is replaced by the medium containing 10% CCK-8 and cells are incubated at 37°C for an additional 2 h. The absorbance is then measured at a wavelength of 450 nm on an ELX800 absorbance microplate reader. |
| Animal Admin | Wildtype male C57BL/6 mice are fed with a HFD consisting of 45% of calories from fat starting at eight weeks of age for 12-24 weeks, while ND C57BL/6 controls are maintained on normal chow diet consisting of 4.5% fat. C57BL/6 diets are fed containing ω-3 fatty acids. Rosiglitazone treatment is administered for three weeks by addition of the compound to the diet in mice that have been on HFD for 16 weeks. Each mouse consumes on average 3.5 mg per kg rosiglitazone per day. AmLexanox is administered by daily oral gavage. For the prevention groups, amLexanox (25 mg per kg or 100 mg per kg) administration is begun concurrently with HFD feeding at eight weeks of age. For the treatment groups, 25 mg per kg amLexanox treatment is begun at 20 weeks of age after 12 weeks of HFD. To test the effect of amLexanox withdrawal, mice in the treatment group are switched from amLexanox gavage to vehicle control after eight weeks of amLexanox treatment. Control and ob/ob mice are fed with a normal chow diet and gavaged with 100 mg per kg amLexanox or vehicle control beginning at ten weeks of age. Animals are housed in a specific pathogen-free facility with a 12-hour light/12-hour dark cycle and given free access to food and water. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 570.0±50.0 °C at 760 mmHg |
| Melting Point | >3000C |
| Molecular Formula | C16H14N2O4 |
| Molecular Weight | 298.293 |
| Flash Point | 298.5±30.1 °C |
| Exact Mass | 298.095367 |
| PSA | 106.42000 |
| LogP | 3.74 |
| Vapour Pressure | 0.0±1.6 mmHg at 25°C |
| Index of Refraction | 1.669 |
| Storage condition | -20°C Freezer |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Precautionary Statements | P301 + P312 + P330 |
| Hazard Codes | Xn,Xi |
| Risk Phrases | R22:Harmful if swallowed. R36/37/38:Irritating to eyes, respiratory system and skin . |
| Safety Phrases | S26 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | XZ3012000 |
| HS Code | 2934999090 |
|
~87% 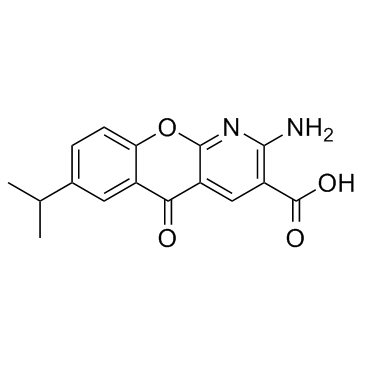
68302-57-8 |
| Literature: Nohara; Ishiguro; Ukawa; Sugihara; Maki; Sanno Journal of Medicinal Chemistry, 1985 , vol. 28, # 5 p. 559 - 586 |
|
~% 
68302-57-8 |
| Literature: Journal of Medicinal Chemistry, , vol. 28, # 5 p. 559 - 586 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |