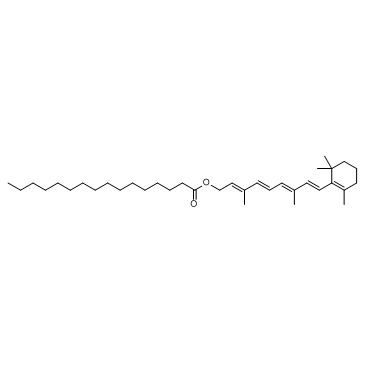
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
VH6825000
-
CHEMICAL NAME :
-
Retinol, acetate
-
CAS REGISTRY NUMBER :
-
127-47-9
-
BEILSTEIN REFERENCE NO. :
-
1915439
-
LAST UPDATED :
-
199701
-
DATA ITEMS CITED :
-
24
-
MOLECULAR FORMULA :
-
C22-H32-O2
-
MOLECULAR WEIGHT :
-
328.54
-
WISWESSER LINE NOTATION :
-
L6UTJ A1 B1U1Y1&U2U1Y1&U2OV1 C1 C1
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
4100 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
432 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
6352 ku/kg/13W-C
-
TOXIC EFFECTS :
-
Liver - changes in liver weight Blood - changes in serum composition (e.g. TP, bilirubin, cholesterol) Nutritional and Gross Metabolic - weight loss or decreased weight gain
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
6347 mg/kg/21D-I
-
TOXIC EFFECTS :
-
Blood - changes in other cell count (unspecified) Blood - changes in platelet count Related to Chronic Data - death
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
2208 mg/kg/21D-I
-
TOXIC EFFECTS :
-
Blood - pigmented or nucleated red blood cells Blood - changes in erythrocyte (RBC) count Blood - changes in platelet count
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
51800 mg/kg/2Y-C
-
TOXIC EFFECTS :
-
Tumorigenic - neoplastic by RTECS criteria Skin and Appendages - tumors
-
TYPE OF TEST :
-
TD - Toxic dose (other than lowest)
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
95 gm/kg/2Y-C
-
TOXIC EFFECTS :
-
Tumorigenic - neoplastic by RTECS criteria Endocrine - thyroid tumors Skin and Appendages - tumors
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
480 mg/kg
-
SEX/DURATION :
-
female 6-19 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - live birth index (measured after birth) Reproductive - Effects on Newborn - viability index (e.g., # alive at day 4 per # born alive) Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
310 mg/kg
-
SEX/DURATION :
-
female 10-12 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - other effects to embryo
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1377 mg/kg
-
SEX/DURATION :
-
female 15-19 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - respiratory system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1377 mg/kg
-
SEX/DURATION :
-
female 15-19 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - stillbirth
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
826 mg/kg
-
SEX/DURATION :
-
female 12-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - cytological changes (including somatic cell genetic material)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1205 mg/kg
-
SEX/DURATION :
-
female 6-19 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - viability index (e.g., # alive at day 4 per # born alive)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
310 mg/kg
-
SEX/DURATION :
-
female 9-11 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - Central Nervous System Reproductive - Specific Developmental Abnormalities - eye/ear Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue)
MUTATION DATA
-
TYPE OF TEST :
-
DNA inhibition
-
TEST SYSTEM :
-
Rodent - mouse Ascites tumor
-
DOSE/DURATION :
-
100 umol/L
-
REFERENCE :
-
ONCOBS Oncology. (S. Karger AG, Postfach CH-4009 Basel, Switzerland) V.21- 1967- Volume(issue)/page/year: 44,356,1987 *** REVIEWS *** TOXICOLOGY REVIEW NTIS** National Technical Information Service. (Springfield, VA 22161) Formerly U.S. Clearinghouse for Scientific & Technical Information. Volume(issue)/page/year: PB-275-754 *** NIOSH STANDARDS DEVELOPMENT AND SURVEILLANCE DATA *** NIOSH OCCUPATIONAL EXPOSURE SURVEY DATA : NOHS - National Occupational Hazard Survey (1974) NOHS Hazard Code - 80572 No. of Facilities: 277 (estimated) No. of Industries: 4 No. of Occupations: 11 No. of Employees: 5119 (estimated) NOES - National Occupational Exposure Survey (1983) NOES Hazard Code - 80572 No. of Facilities: 550 (estimated) No. of Industries: 6 No. of Occupations: 20 No. of Employees: 13296 (estimated) No. of Female Employees: 7092 (estimated)
|




![[(E)-3-methyl-4-oxobut-2-enyl] acetate structure](https://image.chemsrc.com/caspic/338/26586-02-7.png)



![(1E,3E)-3-methyl-1-[2,6,6-trimethylcyclohex-1-enyl]hexa-1,3,5-triene structure](https://image.chemsrc.com/caspic/027/43219-55-2.png)