CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
VH6750000
-
CHEMICAL NAME :
-
Retinol, all trans-
-
CAS REGISTRY NUMBER :
-
68-26-8
-
BEILSTEIN REFERENCE NO. :
-
0403040
-
LAST UPDATED :
-
199706
-
DATA ITEMS CITED :
-
60
-
MOLECULAR FORMULA :
-
C20-H30-O
-
MOLECULAR WEIGHT :
-
286.50
-
WISWESSER LINE NOTATION :
-
L6UTJ A1 B1U1Y1&U2U1Y1&U2Q C1 C1
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - child
-
DOSE/DURATION :
-
5475 ku/kg/1Y-I
-
TOXIC EFFECTS :
-
Lungs, Thorax, or Respiration - pleural effusion Gastrointestinal - other changes Musculoskeletal - other changes
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
2 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
1510 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
1510 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
73 mL/kg/16D-I
-
TOXIC EFFECTS :
-
Musculoskeletal - other changes Biochemical - Enzyme inhibition, induction, or change in blood or tissue levels - phosphatases Related to Chronic Data - death
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
73 mL/kg/16D-I
-
TOXIC EFFECTS :
-
Musculoskeletal - other changes Nutritional and Gross Metabolic - changes in calcium Biochemical - Enzyme inhibition, induction, or change in blood or tissue levels - phosphatases
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
68 mg/kg
-
SEX/DURATION :
-
female 1-39 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - urogenital system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
200 mg/kg
-
SEX/DURATION :
-
female 8 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - eye/ear
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
76560 ug/kg
-
SEX/DURATION :
-
female 8-10 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain) Reproductive - Effects on Newborn - behavioral
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
113 mg/kg
-
SEX/DURATION :
-
female 10 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
150 mg/kg
-
SEX/DURATION :
-
female 7-10 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - Central Nervous System Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
683 mg/kg
-
SEX/DURATION :
-
female 4-16 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Fertility - other measures of fertility Reproductive - Effects on Embryo or Fetus - fetal death
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
22 mg/kg
-
SEX/DURATION :
-
female 1-22 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - weaning or lactation index (e.g., # alive at weaning per # alive at day 4) Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
240 mg/kg
-
SEX/DURATION :
-
female 9 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Effects on Embryo or Fetus - extra-embryonic structures (e.g., placenta, umbilical cord)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1200 ku/kg
-
SEX/DURATION :
-
female 6-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue) Reproductive - Effects on Newborn - viability index (e.g., # alive at day 4 per # born alive) Reproductive - Effects on Newborn - behavioral
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
60060 ug/kg
-
SEX/DURATION :
-
female 10 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system Reproductive - Specific Developmental Abnormalities - other developmental abnormalities
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
60 mg/kg
-
SEX/DURATION :
-
female 10 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - eye/ear
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
60 mg/kg
-
SEX/DURATION :
-
female 10 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
30 mg/kg
-
SEX/DURATION :
-
female 11 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Parenteral
-
DOSE :
-
150 mg/kg
-
SEX/DURATION :
-
female 10 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
72 mg/kg
-
SEX/DURATION :
-
female 11-13 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
150 mg/kg
-
SEX/DURATION :
-
female 9 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system Reproductive - Specific Developmental Abnormalities - other developmental abnormalities
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
30 mg/kg
-
SEX/DURATION :
-
female 3 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - skin and skin appendages
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
120 mg/kg
-
SEX/DURATION :
-
female 12 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
9610 mg/kg
-
SEX/DURATION :
-
female 8-12 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - live birth index (measured after birth) Reproductive - Effects on Newborn - viability index (e.g., # alive at day 4 per # born alive) Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
30 mg/kg
-
SEX/DURATION :
-
lactating female 1 day(s) post-birth
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - skin and skin appendages
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
375 mg/kg
-
SEX/DURATION :
-
female 7-11 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
45045 ug/kg
-
SEX/DURATION :
-
female 8 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - eye/ear
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
145 mg/kg
-
SEX/DURATION :
-
female 8 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Fertility - litter size (e.g. # fetuses per litter; measured before birth) Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
145 mg/kg
-
SEX/DURATION :
-
female 9 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - Central Nervous System Reproductive - Specific Developmental Abnormalities - eye/ear Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
145 mg/kg
-
SEX/DURATION :
-
female 10 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetal death Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
180 mg/kg
-
SEX/DURATION :
-
female 10 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - body wall Reproductive - Specific Developmental Abnormalities - gastrointestinal system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
96 mg/kg
-
SEX/DURATION :
-
female 11-14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue) Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
480 mg/kg
-
SEX/DURATION :
-
female 11-14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetal death
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
12 mg/kg
-
SEX/DURATION :
-
female 8 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - Central Nervous System Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
DOSE :
-
480 mg/kg
-
SEX/DURATION :
-
female 10-13 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue) Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
DOSE :
-
120 mg/kg
-
SEX/DURATION :
-
female 8 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
DOSE :
-
240 mg/kg
-
SEX/DURATION :
-
female 14-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - hepatobiliary system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
45 mg/kg
-
SEX/DURATION :
-
female 12 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue) Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
77 mg/kg
-
SEX/DURATION :
-
female 8-18 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetal death Reproductive - Specific Developmental Abnormalities - other developmental abnormalities
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
52550 ug/kg
-
SEX/DURATION :
-
female 9-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants)
-
TYPE OF TEST :
-
DNA inhibition
-
TYPE OF TEST :
-
DNA inhibition
MUTATION DATA
-
TYPE OF TEST :
-
Sister chromatid exchange
-
TEST SYSTEM :
-
Rodent - hamster Cells - not otherwise specified
-
DOSE/DURATION :
-
24 mg/L
-
REFERENCE :
-
MUREAV Mutation Research. (Elsevier Science Pub. B.V., POB 211, 1000 AE Amsterdam, Netherlands) V.1- 1964- Volume(issue)/page/year: 246,67,1991 *** REVIEWS *** TOXICOLOGY REVIEW CLPTAT Clinical Pharmacology and Therapeutics (St. Louis). (C.V. Mosby Co., 11830 Westline Industrial Dr., St. Louis, MO 63146) V.1- 1960- Volume(issue)/page/year: 5,480,1964 TOXICOLOGY REVIEW ARVPAX Annual Review of Pharmacology. (Palo Alto, CA) V.1-15, 1961-75. For publisher information, see ARPTDI. Volume(issue)/page/year: 5,447,1965 TOXICOLOGY REVIEW EVHPAZ EHP, Environmental Health Perspectives. (U.S. Government Printing Office, Supt of Documents, Washington, DC 20402) No.1- 1972- Volume(issue)/page/year: 15,121,1976 TOXICOLOGY REVIEW CRTXB2 CRC Critical Reviews in Toxicology. (CRC Press, Inc., 2000 Corporate Blvd., NW, Boca Raton, FL 33431) V.1- 1971- Volume(issue)/page/year: 6,351,1979 TOXICOLOGY REIVEW NTIS** National Technical Information Service. (Springfield, VA 22161) Formerly U.S. Clearinghouse for Scientific & Technical Information. Volume(issue)/page/year: PB-275-754 TOXICOLOGY REVIEW JACTDZ Journal of the American College of Toxicology. (Mary Ann Liebert, Inc., 1651 Third Ave., New York, NY 10128) V.1-12, 1982-1993. Discontinued. Volume(issue)/page/year: 6(3),279,1987 *** NIOSH STANDARDS DEVELOPMENT AND SURVEILLANCE DATA *** NIOSH OCCUPATIONAL EXPOSURE SURVEY DATA : NOES - National Occupational Exposure Survey (1983) NOES Hazard Code - X1676 No. of Facilities: 546 (estimated) No. of Industries: 3 No. of Occupations: 7 No. of Employees: 12809 (estimated) No. of Female Employees: 9075 (estimated)
|




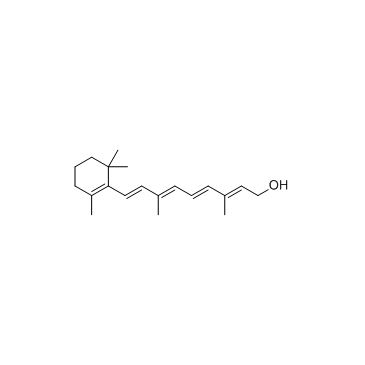

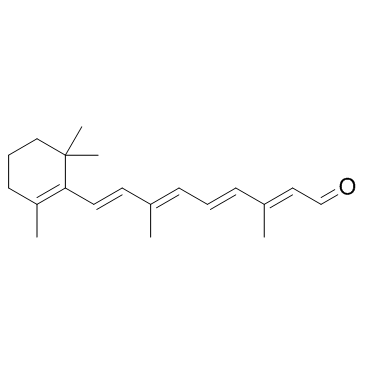
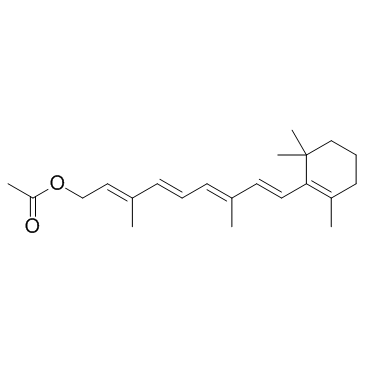
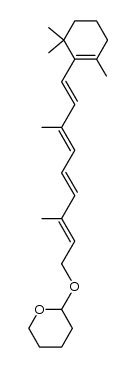



![(1E,3E)-3-methyl-1-[2,6,6-trimethylcyclohex-1-enyl]hexa-1,3,5-triene structure](https://image.chemsrc.com/caspic/027/43219-55-2.png)
