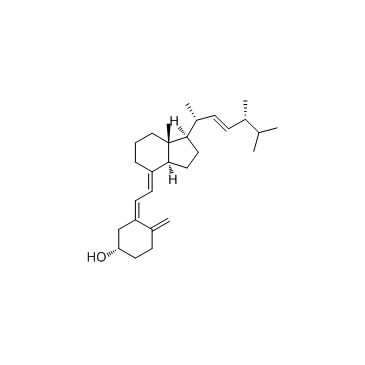
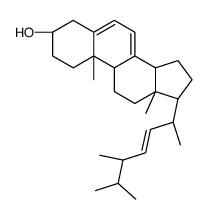
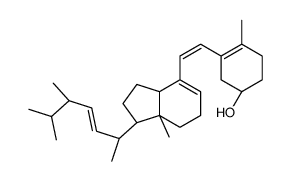
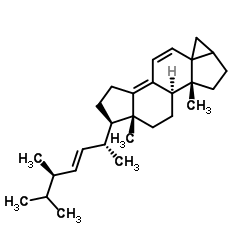
57-87-4
| Name | ergosterol |
|---|---|
| Synonyms |
Ergosterol
EINECS 200-352-7 (24R)-Ergosta-5,7,22-trien-3b-ol (3S,9S,10R,13R,14R,17R)-17-[(2R,3E,5R)-5,6-Dimethyl-3-hepten-2-yl]-10,13-dimethyl-2,3,4,9,10,11,12,13,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-ol PROVITAMIN D2 ERGOSTERINE (22E)-Ergosta-5,7,22-trien-3β-ol ERGOSTERIN Ergosta-5,7,22-trien-3β-ol PROVITAMINE D2 MFCD00003623 (3β)-Ergosta-5,7,22-trien-3-ol (3b,22E)-Ergosta-5,7,22-trien-3-ol (3β,2E)-Ergosta-5,7,22-trien-3-ol provitamind (3β,22E)-Ergosta-5,7,22-trien-3-ol Ergosta-5:6,7:8,22:23-trien-3-ol |
| Description | Ergosterol is the primary sterol found in fungi, with antioxidative, anti-proliferative, and anti-inflammatory effects. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| In Vitro | Ergosterol is a sterol isolated from Grifola frondosa, which can be used in the research of mast cell-dependent allergic diseases. Ergosterol (10, 20, 50 μM) inhibits the antigen-induced release of β-hexosaminidase and histamine in antigen-stimulated RBL-2H3 cells. Ergosterol (20 and 50 μM) significantly reduces the mRNA levels of of IL-4 and TNF-α. Ergosterol (50 μM) inhibits the antigen-induced aggregation of FcεRI[1]. |
| In Vivo | Ergosterol (25, 50 mg/kg, p.o.) significantly mitigates the reduced cardiac performance in rats induced by LPS, increases SOD activity and decreases the formation of MDA, CK-MB, and LDH in LPS-induced sepsis rats[2]. |
| Cell Assay | RBL-2H3 cells are seeded into 24-well plates containing gelatin-coated cover glasses at 0.75 × 105 cells/well and sensitized with anti-DNP IgE. After culture for 24 hours, the cells cultured on cover glasses are pretreated with or without 50 μM Ergosterol or 1 mM methyl beta cyclodextrin (MβCD) in PIPES buffer for 20 minutes. The cells are then challenged with DNP-HSA (50 ng/mL) for 20 minutes. After washing with ice-cold PBS immediately, the cells are fixed with 3.7% formaldehyde in PBS for 20 minutes and blocked with 1% BSA in PBS. The IgE/α-chain of FcεRI complexes on cell surfaces are detected using goat anti-mouse IgE antiserum, and Alexa Fluor 488-conjugated anti-goat IgG. Fluorescence images are acquired using a laser scanning confocal microscope with Zen software. The data are quantified by counting the aggregation number of FcεRI positive cells and presented as aggregation positive cells/total cells. The cells are counted in six independent micrographs for each sample[1]. |
| Animal Admin | Rats[2] Experimental myocardial injury in rats is performed by LPS injection (15 mg/kg). Dexmedetomidine (Dex) is used as a positive control. The experimental animals are randomly divided into five groups (n = 10) as follows: Control group, rats receive 2% gum acacia suspension orally at a dose of 2 mL/kg for 5 days, followed by normal saline injected intraperitoneally on day 5; LPS group, rats receive 2% gum acacia suspension at dose of 2 mL/kg for 5 days with LPS simultaneously injected intraperitoneally day 5; LPS+ Dex group, rats are treated with 2 mg/kg Dex suspension followed by LPS injection on day 5; LPS + Ergosterol (25 mg/kg, 50 mg/kg) groups, 25 or 50 mg/kg Ergosterol are given to rats orally for 5 consecutive days, and LPS is injected on day 5. Twelve hours after LPS treatment, blood samples are collected through the retro-orbital plexus. The serum specimens are centrifugated at 4, 000 × g for 15 min and stored at −80°C until needed. Thereafter, rats are anesthetized and sacrificed. Heart tissues are removed and homogenized in ice-cold phosphate buffered saline (50 mM, pH 7.4). Heart tissue homogenates from different groups are centrifuged at 12, 000 × g for 45 min at 4°C and the supernatants retained for further biochemical evaluations[2]. |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 501.5±39.0 °C at 760 mmHg |
| Melting Point | 156-158 °C(lit.) |
| Molecular Formula | C28H44O |
| Molecular Weight | 396.648 |
| Flash Point | 216.3±19.3 °C |
| Exact Mass | 396.339203 |
| PSA | 20.23000 |
| LogP | 9.30 |
| Vapour Pressure | 0.0±2.9 mmHg at 25°C |
| Index of Refraction | 1.543 |
| Water Solubility | PRACTICALLY INSOLUBLE |
| Symbol |


GHS06, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H302-H315-H319-H331-H336-H351-H361d-H372 |
| Precautionary Statements | P261-P281-P305 + P351 + P338-P311 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face respirator (US);Gloves;multi-purpose combination respirator cartridge (US) |
| Hazard Codes | T+:Verytoxic; |
| Risk Phrases | R28 |
| Safety Phrases | S28-S36/37-S45 |
| RIDADR | UN 2811 6.1/PG 2 |
| WGK Germany | 3 |
| Packaging Group | II |
| Hazard Class | 6.1 |
| Precursor 0 | |
|---|---|
| DownStream 9 | |