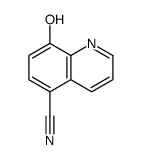
5852-78-8
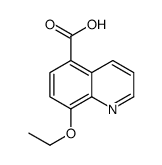
| Name | 8-hydroxyquinoline-5-carboxylic acid |
|---|---|
| Synonyms |
8XQ
8-Hydroxy-chinolin-5-carbonsaeure 8-hydroxy-quinoline-5-carboxylic acid UNII-JM015YQC1C 5-Carboxy-8-hydroxyquinoline IOX1 8-Hydroxy-5-quinolinecarboxylic acid |
| Description | IOX1 is the most potent broad-spectrum inhibitor of 2OG oxygenases, including the JmjC demethylases; IC50 for KDM4A/KDM3A is 0.6/0.1 uM.IC50 value: 0.6/0.1 uM(KDM4A/KDM3A) [1]Target: JmjC KDMs inhibitorIOX1 is the most potent against a representative panel of 2OG oxygenases, including non-JmjC 2OG oxygenases, with an in vitro IC50 value in the micromolar range. However, its efficacy in cells is about a hundred-fold lower (HeLa cells, KDM4A, IC50 = 86uM), possibly due to low cell permeability resulting from itspolar C-5 carboxyl group. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 464.5±30.0 °C at 760 mmHg |
| Molecular Formula | C10H7NO3 |
| Molecular Weight | 189.167 |
| Flash Point | 234.7±24.6 °C |
| Exact Mass | 189.042587 |
| PSA | 70.42000 |
| LogP | 1.81 |
| Appearance | white to brown |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.730 |
| Storage condition | 2-8°C |
| Water Solubility | DMSO: soluble10mg/mL, clear |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Hazard Codes | Xn |
| Risk Phrases | 22 |
| RIDADR | NONH for all modes of transport |
| HS Code | 2933499090 |
|
~51% 
5852-78-8 |
| Literature: Schiller, Rachel; Scozzafava, Giuseppe; Tumber, Anthony; Wickens, James R.; Bush, Jacob T.; Rai, Ganesha; Lejeune, Clarisse; Choi, Hwanho; Yeh, Tzu-Lan; Chan, Mun Chiang; Mott, Bryan T.; McCullagh, James S. O.; Maloney, David J.; Schofield, Christopher J.; Kawamura, Akane ChemMedChem, 2014 , vol. 9, # 3 p. 566 - 571 |
|
~49% 
5852-78-8 |
| Literature: Sosic, Izidor; Mirkovic, Bojana; Arenz, Katharina; Stefane, Bogdan; Kos, Janko; Gobec, Stanislav Journal of Medicinal Chemistry, 2013 , vol. 56, # 2 p. 521 - 533 |
|
~% 
5852-78-8 |
| Literature: Lippmann; Fleissner Chemische Berichte, 1886 , vol. 19, p. 2468 Monatshefte fuer Chemie, 1887 , vol. 8, p. 311 Full Text Show Details v. Niementowski; Sucharda Chemische Berichte, 1916 , vol. 49, p. 16 |
|
~% 
5852-78-8 |
| Literature: Matsumura Journal of the American Chemical Society, 1935 , vol. 57, p. 124,127 |
|
~% 
5852-78-8 |
| Literature: Matsumura Journal of the American Chemical Society, 1935 , vol. 57, p. 124,127 |
|
~% 
5852-78-8 |
| Literature: v. Niementowski; Sucharda Chemische Berichte, 1916 , vol. 49, p. 16 |
|
~% 
5852-78-8 |
| Literature: Clemo; Howe Journal of the Chemical Society, 1955 , p. 3552 |
|
~% 
5852-78-8 |
| Literature: Clemo; Howe Journal of the Chemical Society, 1955 , p. 3552 |
|
~% 
5852-78-8 |
| Literature: Clemo; Howe Journal of the Chemical Society, 1955 , p. 3552 |
| Precursor 7 | |
|---|---|
| DownStream 3 | |
| HS Code | 2933499090 |
|---|---|
| Summary | 2933499090. other compounds containing in the structure a quinoline or isoquinoline ring-system (whether or not hydrogenated), not further fused. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |