93221-48-8
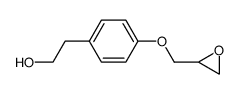
| Name | (2S)-1-[4-[2-(cyclopropylmethoxy)ethyl]phenoxy]-3-(propan-2-ylamino)propan-2-ol |
|---|---|
| Synonyms |
(S)-(-)-Betaxolol
Levobetaxolol (S)-(-)-3-<4-<2-(cyclopropylmethoxy)ethyl>phenoxy>-1-(isopropylamino)-2-propanol UNII-75O9XHA4TU (-)-Betaxolol Levobetaxolol [INN] (S)-(-)-3-[4-[2-(cyclopropylmethoxy)]ethyl]phenoxy-1-(N-isopropylamino)-propan-2-ol |
| Description | Levobetaxolol is a potent and high affinity β-adrenergic antagonist with IC50 values of 33.2, 2970, 709 nM for guinea pig atrial β1, tracheal β2 and rat colonic β3 receptors, respectively. Levobetaxolol reduces IOP (intraocular pressure). Levobetaxolol exhibits a micromolar affinity for L-type Ca21-channels. Levobetaxolol decreases the effects of ischaemia/reperfusion injury in rats. Levobetaxolol has the potential for the research of glaucoma[1][2]. |
|---|---|
| Related Catalog | |
| Target |
β1 adrenoceptor:33.2 nM (IC50) β2 adrenoceptor:2970 nM (IC50) β3 adrenoceptor:709 nM (IC50) |
| In Vitro | Levobetaxolol shows a higher affinity at cloned human β1 and β2 receptor with Ki values of 0.76, 32.6 nM, respectively[1]. Levobetaxolol inhibits functional activities in cells expressing human recombinant β1 and β2 receptors with Kb values of 6, 39 nM, respectively[1]. |
| References |
| Density | 1.067g/cm3 |
|---|---|
| Boiling Point | 448ºC at 760 mmHg |
| Melting Point | 71-72ºC |
| Molecular Formula | C18H29NO3 |
| Molecular Weight | 307.42800 |
| Flash Point | 224.7ºC |
| Exact Mass | 307.21500 |
| PSA | 50.72000 |
| LogP | 2.78430 |
| Index of Refraction | 1.529 |
| Precursor 10 | |
|---|---|
| DownStream 0 | |
![S-(-)-1-{4-[2-(allyloxy)-ethyl]phenoxy}-3-isopropylamino propan-2-ol structure](https://image.chemsrc.com/caspic/493/874112-86-4.png)
![(S)-2-[4-(2-cyclopropylmethoxy ethyl)phenoxymethyl]oxirane structure](https://image.chemsrc.com/caspic/114/874809-42-4.png)