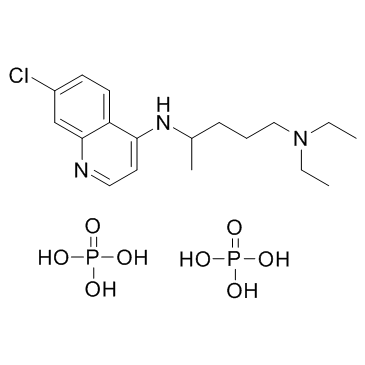
50-63-5
| Name | Chloroquine Diphosphate |
|---|---|
| Synonyms |
MFCD00069852
acide phosphorique - N-(7-chloroquinoléin-4-yl)-N,N-diéthylpentane-1,4-diamine (2:1) Chloroquine phosphate 7-Chloro-4-[4-(diethylamino)-1-methylbutylamino]quinoline Diphosphate EINECS 200-055-2 N-(7-chloroquinolin-4-yl)-N,N-diethylpentane-1,4-diamine bis(phosphate) N4-(7-chloroquinolin-4-yl)-N1,N1-diethylpentane-1,4-diamine phosphate (1:2) N-(7-Chloro-4-quinolinyl)-N,N-diethyl-1,4-pentanediamine phosphate (1:2) N4-(7-Chloro-4-quinolyl)-N1,N1-diethyl-1,4-pentanediamine Diphosphate Chloroquine diphosphate salt 7-Chloro-4-((4-(diethylamino)-1-methylbutyl)amino)quinoline phosphate (1:2) Aralen phosphate 1,4-Pentanediamine, N-(7-chloro-4-quinolinyl)-N,N-diethyl-, phosphate (1:2) 1,4-pentanediamine, N4-(7-chloro-4-quinolinyl)-N1,N1-diethyl-, phosphate (1:2) N-(7-Chloroquinolin-4-yl)-N,N-diethylpentane-1,4-diamine phosphate (1:2) Chloroquine diphosphate Imagon Tresochin Chloroquinediphosphate Malaquin Phosphorosäure--N-(7-chlorchinolin-4-yl)-N,N-diethylpentan-1,4-diamin(2:1) |
| Description | Chloroquine (diphosphate) is an antimalarial and anti-inflammatory drug widely used to treat malaria and rheumatoid arthritis. Chloroquine is an inhibitor of autophagy and toll-like receptors (TLRs). |
|---|---|
| Related Catalog | |
| Target |
Autophagy, TLRs[1][2][3] |
| In Vitro | Chloroquine (CHQ, 20 μM) inhibits IL-12p70 release and reduces Th1-priming capacity of activated human monocyte-derived Langerhans-like cells (MoLC). Chloroquine (CHQ, 20 μM) enhances IL-1–induced IL-23 secretion in MoLC and subsequently increases IL-17A release by primed CD4+ T cells[1]. Chloroquine (25 μM) suppresses MMP-9 mRNA expression in normoxia and hypoxia in parental MDA-MB-231 cells. Chloroquine has cell-, dose- and hypoxia-dependent effects on MMP-2, MMP-9 and MMP-13 mRNA expression[2]. TLR7 and TLR9 inhibition using IRS-954 or chloroquine significantly reduces HuH7 cell proliferation in vitro[3]. |
| In Vivo | Chloroquine (80 mg/kg, i.p.) does not prevent the growth of the triple-negative MDA-MB-231 cells with high or low TLR9 expression levels in the orthotopic mouse model[2]. TLR7 and TLR9 inhibition using IRS-954 or chloroquine significantly inhibits tumour growth in the mouse xenograft model. HCC development in the DEN/NMOR rat model is also significantly inhibited by chloroquine[3]. |
| Cell Assay | The cells are cultured in 6-well plates with normal culture medium in the presence of vehicle or 25 or 50 μM chloroquine, until near confluency, after which they are rinsed with sterile phosphate-buffered saline (PBS) and cultured further for the indicated times in serum-free culture medium. At the desired time-points, the culture medium is discarded and the cells are quickly harvested in lysis buffer and clarified by centrifugation. Subsequent to boiling the supernatants in reducing sodium dodecyl sulphate (SDS) sample buffer, equal amounts of protein (100 μg) are loaded per lane and the samples are electrophoresed into 10 or 4-20% gradient polyacrylamide SDS gels, then transferred to a nitrocellulose membrane. To detect TLR9, the blots are incubated overnight at 4°C with anti-TLR9 antibodies, diluted 1:500 in Tris-buffered saline with 0.1% (v/v) Tween-20 (TBST). Equal loading is confirmed with polyclonal rabbit anti-actin. Secondary detection is performed with horseradish peroxidase-linked secondary antibodies. The protein bands are visualized by chemiluminescence using an ECL kit. |
| Animal Admin | Control and TLR9 siRNA MDA-MB-231 cells (5×105 cells in 100 μL) are inoculated into the mammary fat pads of four-week-old, immune-deficient mice (athymic nude/nu Foxn1). Treatments are started seven days after tumor cell inoculation. The mice are treated daily either with intraperitoneal (i.p.) chloroquine (80 mg/kg) or vehicle (PBS). The animals are monitored daily for clinical signs. Tumor measurements are performed twice a week and tumor volume is calculated according to the formula V=(π/6) (d1×d2)3/2, where d1 and d2 are perpendicular tumor diameters. The tumors are allowed to grow for 22 days, at which point the mice are sacrificed and the tumors are dissected for a final measurement. Throughout the experiments, the animals are maintained under controlled pathogen-free environmental conditions (20-21°C, 30-60% relative humidity and a 12-h lighting cycle). The mice are fed with small-animal food pellets and supplied with sterile water ad libitum. |
| References |
| Boiling Point | 460.6ºC at 760 mmHg |
|---|---|
| Melting Point | 200 °C (dec.)(lit.) |
| Molecular Formula | C18H32ClN3O8P2 |
| Molecular Weight | 515.862 |
| Flash Point | 232.3ºC |
| Exact Mass | 515.135315 |
| PSA | 203.30000 |
| LogP | 3.02640 |
| Storage condition | Desiccate at RT |
| Water Solubility | H2O: 50 mg/mL, clear |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Precautionary Statements | P301 + P312 + P330 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn:Harmful; |
| Risk Phrases | R22 |
| Safety Phrases | S22-S24/25 |
| RIDADR | 1544 |
| WGK Germany | 3 |
| RTECS | VB2450000 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 2933499090 |
| Precursor 0 | |
|---|---|
| DownStream 1 | |
| HS Code | 2933499090 |
|---|---|
| Summary | 2933499090. other compounds containing in the structure a quinoline or isoquinoline ring-system (whether or not hydrogenated), not further fused. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |