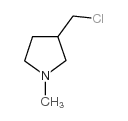
1982-37-2
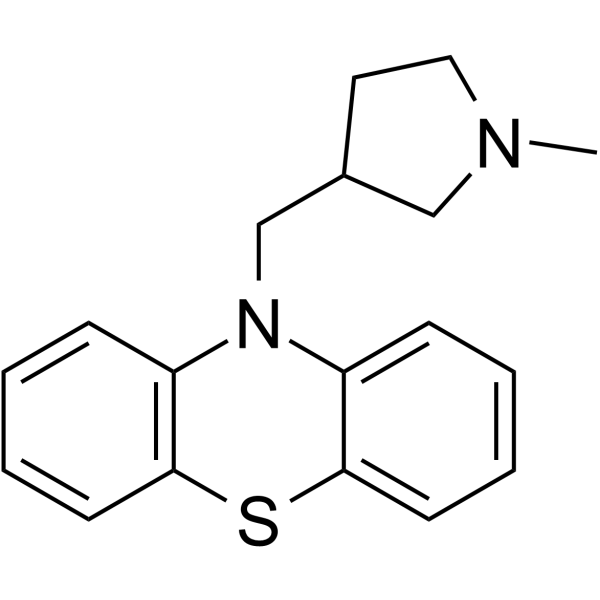
| Name | 10-[(1-methylpyrrolidin-3-yl)methyl]phenothiazine |
|---|---|
| Synonyms |
10-[(1-methyl-3-pyrrolidinyl)methyl]phenothiazine
Tacazyl 10-(1-methyl-pyrrolidin-3-ylmethyl)-phenothiazine 10-(1-Methyl-pyrrolidin-3-ylmethyl)-phenothiazin 10-<1-Methyl-pyrrolidinyl-(3)-methyl>-phenothiazin. methdilazine Methdilazinum Metodilazina Tacryl Methodilazine Methdilazinum [INN-Latin] Disyncram Tacaryl Metodilazina [INN-Spanish] 10-(1-methyl-pyrrolidin-3-ylmethyl)-10H-phenothiazine |
| Description | Methdilazine is an orally active antibiotic (histamine antagonist). Methdilazine can inhibit various mycobacterium with MIC values at 5-15 μg/mL in vitro and in vivo, which can be used for the research of infectious diseases[1][2]. |
|---|---|
| Related Catalog | |
| Target |
MIC: 5-15 μg/mL (mycobacterium) |
| In Vitro | Methdilazine (0-20 μg/mL approximately, 18 h) inhibits kinds of mycobacterium with MIC values ranging from 5 μg/mL to 15 μg/mL[1]. Cell Proliferation Assay[1] Cell Line: mycobacterium: M. smegmatis 798/1546, M.,fortuitum 1529, M. scrofulaceum 1323, M. gordonae 1324, M. rnarinum 50, M.,flavescens 1541, M. terrae 1450, M. tuberculosis, H37Ra 16, H37Rv 16, K1, K2, ICRC bacillus,'Skinsnes' bacillus. Concentration: 0-20 μg/mL approximately Incubation Time: 18 h Result: Inhibited mycobacterium with MIC values ranging from 5 μg/mL to 15 μg/mL. |
| In Vivo | Methdilazine (Intraperitoneal injection, 10 μg/gm body wt/day, 6 weeks) is antagonistic to mycobacteria in H37Rv infected mice[1]. Methdilazine (Oral administration, 10 mg/kg per day, 28 days) improves survival of Mycobacterium Tuberculosis (Mtb) H37Rv infected mice[2]. Animal Model: H37Rv infected mice[1] Dosage: 10 μg/gm body wt/day, 6 weeks Administration: Intraperitoneal injection Result: Displayed an anti-mycobacterial activity to mycobacteria. Animal Model: Mycobacterium Tuberculosis (Mtb) H37Rv infected Swiss albino male mice[2] Dosage: 10 mg/kg per day for 28 days Administration: Oral administration Result: Increased surviving time to 28 days with no sign of disease, showed 71.42% survival. |
| References |
| Density | 1.185 g/cm3 |
|---|---|
| Boiling Point | 430.4ºC at 760 mmHg |
| Molecular Formula | C18H20N2S |
| Molecular Weight | 296.43000 |
| Flash Point | 214.1ºC |
| Exact Mass | 296.13500 |
| PSA | 31.78000 |
| LogP | 4.24390 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| HS Code | 2934300000 |
|---|
|
~% 
1982-37-2 |
| Literature: Journal of Organic Chemistry, , vol. 26, p. 1529 - 1530 |
|
~% 
1982-37-2 |
| Literature: Arzneimittel Forschung, , vol. 9, p. 715 |
| HS Code | 2934300000 |
|---|---|
| Summary | 2934300000. other compounds containing in the structure a phenothiazine ring-system (whether or not hydrogenated), not further fused. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |