1154-25-2
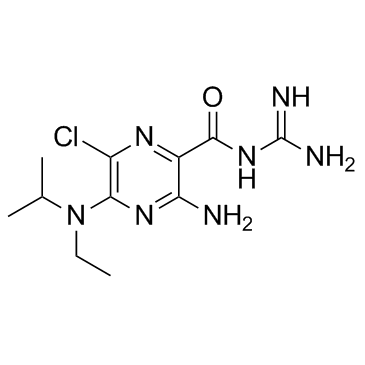
| Name | 5-(N-Ethyl-N-isopropyl) Amiloride |
|---|---|
| Synonyms |
ethylisopropylamiloride
MFCD00151710 3-amino-6-chloro-N-(diaminomethylidene)-5-[ethyl(propan-2-yl)amino]pyrazine-2-carboxamide 3-amino-6-chloro-n-(diaminomethylene)-5-[ethyl(isopropyl)amino]pyrazine-2-carboxamide 3-Amino-6-chloro-N-(diaminomethylene)-5-[ethyl(isopropyl)amino]-2-pyrazinecarboxamide EIPA |
| Description | EIPA is a TRPP3 channel inhibitor with an IC50 of 10.5 μM. EIPA also inhibits Na+/H+-exchanger (NHE) and macropinocytosis. |
|---|---|
| Related Catalog | |
| Target |
IC50: 10.5 μM (TRPP3 channel)[1] NHE[2] Macropinocytosis[3] |
| In Vitro | In the presence of 500 μM amiloride, 100 μM EIPA, 10 μM benzamil, and 10 μM phenamil, 45Ca2+ uptake decreases from 79±9 to 46±4 (58% remaining), 27±4 (34%), 29±5 (37%), and 38±4 (48%) pmol/oocyte/30 min (n=6, P=0.008), respectively. It is found that EIPA, benzamil, and phenamil rapidly and reversibly block Ca2+-activated TRPP3 channel activation at -50 mV, with IC50s of 143±8 (n=36), 10.5±2.2 (n=28), 1.1±0.3 (n=30), and 0.14±0.04 μM (n=25), respectively[1]. The number of autophagic vacuoles increases dramatically in the HAE and HPE groups after EIPA treatment compare with the HAN and HPN groups. EIPA regulates the initiation and maturation of the autophagy associated with amino acids in IEC-18 cells[2]. In addition, the uptake of cinnamoylphenazine (CA-PZ) and neutral red (NR) is inhibited by EIPA[3]. |
| Cell Assay | The effect of EIPA alone (without alanine or proline) is also examined in both control (DMEM cultured cells) and amino acid-starved cells. The cells are incubated for 6 h in DMEM containing 5% FBS and either 0 or 0.3 mM EIPA (labelled QNN and QNE, respectively), HBSS containing either 0 or 0.3 mM EIPA (labelled HNN and HNE, respectively), HBSS with 1.0 mM alanine (labelled HAN) or 0.5 mM proline (labelled HPN), HBSS with 1.0 mM alanine and 0.3 mM EIPA (labelled HAE), and HBSS with 0.5 mM proline and 0.3 mM EIPA (labelled HPE)[2]. |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 543.9±60.0 °C at 760 mmHg |
| Melting Point | 202-205ºC(lit.) |
| Molecular Formula | C11H18ClN7O |
| Molecular Weight | 299.760 |
| Flash Point | 282.8±32.9 °C |
| Exact Mass | 299.126129 |
| PSA | 134.01000 |
| LogP | 4.86 |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.665 |
| Storage condition | 2-8℃ |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | 26-37/39 |
| RIDADR | UN 2811 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 2934999090 |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |