142628-53-3
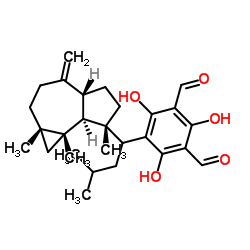
| Name | 5-[(1R)-1-[(1aR,4aR,7S,7aR,7bR)-1,1,7-trimethyl-4-methylidene-1a,2,3,4a,5,6,7a,7b-octahydrocyclopropa[h]azulen-7-yl]-3-methylbutyl]-2,4,6-trihydroxybenzene-1,3-dicarbaldehyde |
|---|---|
| Synonyms |
2,4,6-Trihydroxy-5-{3-methyl-1-[(1aS,4aR,7R,7aS,7bS)-1a,7,7b-trimethyl-4-methylenedecahydro-1H-cyclopropa[e]azulen-7-yl]butyl}isophthalaldehyde
2,4,6-Trihydroxy-5-{(1R)-3-methyl-1-[(1aR,4aR,7S,7aR,7bR)-1,1,7-trimethyl-4-methylenedecahydro-1H-cyclopropa[e]azulen-7-yl]butyl}isophthalaldehyde macrocarpal-C macrocarpal G macrocarpal C |
| Description | Macrocarpal C can be isolated from the 95 % ethanol extract of fresh leaves of E. globulus. Macrocarpal C inhibits the growth of T. mentagrophytes via an increase in the permeability of the fungal membrane. Macrocarpal C increases the production of intracellular ROS and? induces apoptosis as a consequence of DNA fragmentation[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 503.9±50.0 °C at 760 mmHg |
| Molecular Formula | C28H38O5 |
| Molecular Weight | 454.60 |
| Flash Point | 272.6±26.6 °C |
| Exact Mass | 454.271912 |
| PSA | 94.83000 |
| LogP | 10.79 |
| Vapour Pressure | 0.0±1.3 mmHg at 25°C |
| Index of Refraction | 1.594 |
| Hazard Codes | Xi |
|---|