109741-38-0
| Name | 8-[(1E)-3-Hydroxy-3-methyl-1-buten-1-yl]-7-methoxy-2H-chromen-2-o ne |
|---|---|
| Synonyms |
7-ethoxy-3,7-dimethyl-2-octen-1-ol
7-Ethoxygeraniol 8-[(1E)-3-Hydroxy-3-methyl-1-buten-1-yl]-7-methoxy-2H-chromen-2-one |
| Description | Murraol (CM-c2), a coumarin, can be isolated from the leaves of Madagascar pine cork (Apiaceae). Murraol has cyclooxygenase (COX) and lipoxygenase inhibitory properties and has an inhibitory effect on the growth of cancer cells[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 466.9±45.0 °C at 760 mmHg |
| Molecular Formula | C15H16O4 |
| Molecular Weight | 260.285 |
| Flash Point | 176.7±22.2 °C |
| Exact Mass | 260.104858 |
| PSA | 59.67000 |
| LogP | 2.04 |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.604 |
| Hazard Codes | Xi |
|---|
|
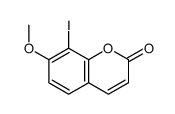
~27% 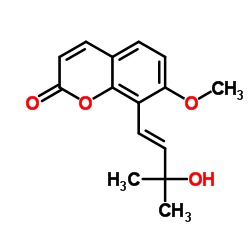
109741-38-0 |
| Literature: Reisch, Johannes; Bathe, Andreas Liebigs Annalen der Chemie, 1988 , p. 543 - 548 |
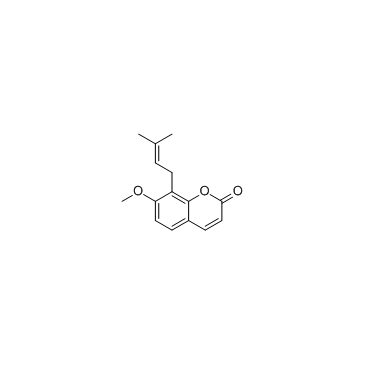
|
~% 
109741-38-0 |
| Literature: Ito; Furukawa Chemical and Pharmaceutical Bulletin, 1989 , vol. 37, # 3 p. 819 - 820 |
| Precursor 3 | |
|---|---|
| DownStream 0 | |