1116235-97-2
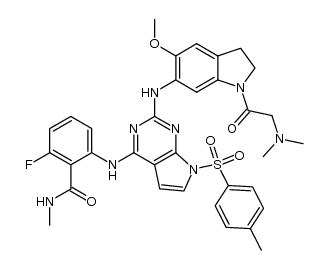
| Name | 2-[[2-[[1-[2-(dimethylamino)acetyl]-5-methoxy-2,3-dihydroindol-6-yl]amino]-7H-pyrrolo[2,3-d]pyrimidin-4-yl]amino]-6-fluoro-N-methylbenzamide |
|---|---|
| Synonyms |
2-[(2-{[1-(N,N-dimethylglycyl)-5-(methyloxy)-2,3-dihydro-1H-indol-6-yl]amino}-1H-pyrrolo[2,3-d]pyrimidin-4-yl)amino]-6-fluoro-N-methylbenzamide
GSK1838705A 2-[(2-{[1-(N,N-Dimethylglycyl)-5-methoxy-2,3-dihydro-1H-indol-6-yl]amino}-1H-pyrrolo[2,3-d]pyrimidin-4-yl)amino]-6-fluoro-N-methylbenzamide pyrrolo[2,3-d]pyrimidine deriv.,40 CS-0695 cc-268 |
| Description | GSK1838705A is a potent and reversible IGF-IR and the insulin receptor inhibitor with IC50s of 2.0 and 1.6 nM, respectively. It also inhibits ALK with an IC50 of 0.5 nM. |
|---|---|
| Related Catalog | |
| Target |
IC50: 2.0 nM (IGF-IR), 1.6 nM (insulin receptor), 0.5 nM (ALK)[1] |
| In Vitro | In cellular phosphorylation assays, GSK1838705A potently inhibits IGF-IR and insulin receptor phosphorylation with IC50s of 85 and 79 nM, respectively. appKi values are 0.7 nM for IGF-IR and 1.1 nM for insulin receptor using the filter binding assay. GSK1838705A inhibits the proliferation in a panel of cell lines derived from solid and hematologic tumors. The EC50s of GSK1838705A range from 20 nM to >8 μM, but are <1 μM in most multiple myeloma and Ewing's sarcoma cell lines[1]. |
| In Vivo | GSK1838705A shows robust antitumor activity in animal xenograft models. Tumor types likely to respond to GSK1838705A include multiple myeloma and Ewing's sarcoma, as well as ALK-driven tumors (e.g., ALCL, NSCLC, and neuroblastoma). A single oral dose of GSK1838705A at 0.1 and 0.3 mg/kg results in 35% and 65% inhibition of IGF-IR phosphorylation, respectively, whereas doses ≥1 mg/kg results in complete inhibition of ligand-induced IGF-IR phosphorylation[1]. |
| Kinase Assay | Baculovirus-expressed glutathione S-transferase–tagged proteins encoding the intracellular domain of IGF-IR (amino acids 957–1367) and IR (amino acids 979–1382) are used for determinations of IC50s by a homogeneous time-resolved fluorescence assay. A filter binding assay is used for appKi determinations using activated IGF-IR and IR kinases. Expanded kinase-selectivity profiling of GSK1838705A is carried out by screening the compound in the KinaseProfiler panel[1]. |
| Cell Assay | Cells are seeded in 96-well dishes, incubated overnight at 37°C, and treated with DMSO or GSK1838705A for 72 h. For the NIH-3T3/LISN proliferation assays, cells are seeded on collagen-coated 96-well tissue culture plates and allowed to adhere for 24 h. The medium is replaced with serum-free medium and the cells are treated with GSK1838705A for 2 h. Cells are incubated for 72 h after addition of IGF-I (30 ng/mL). Cell proliferation is quantified using the CellTiter-Glo Luminescent Cell Viability Assay. IC50s are determined from cytotoxicity curves using a four-parameter curve fit software package[1]. |
| Animal Admin | Mice: Exponentially growing cells are implanted s.c. into the right flank of 8- to 12-wk-old female nu/nu CD-1 or SCID mice. Mice are dosed p.o. with the formulating vehicle or GSK1838705A. Mice are weighed and tumors measured by calipers twice weekly. Tumor volumes are calculated[1] |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Molecular Formula | C27H29FN8O3 |
| Molecular Weight | 532.569 |
| Exact Mass | 532.234680 |
| PSA | 137.46000 |
| LogP | 1.32 |
| Appearance | white to light brown |
| Index of Refraction | 1.700 |
| Storage condition | ?20°C |
| Water Solubility | DMSO: soluble20mg/mL, clear |
| RIDADR | NONH for all modes of transport |
|---|
|
~86% 
1116235-97-2 |
| Literature: SMITHKLINE BEECHAM CORPORATION Patent: WO2009/20990 A1, 2009 ; Location in patent: Page/Page column 360-362 ; WO 2009/020990 A1 |
| Precursor 1 | |
|---|---|
| DownStream 0 | |