2-Bromo-4-fluorobenzoic acid
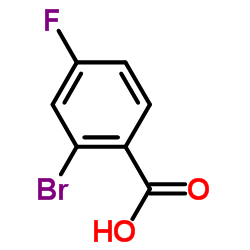
2-Bromo-4-fluorobenzoic acid structure
|
Common Name | 2-Bromo-4-fluorobenzoic acid | ||
|---|---|---|---|---|
| CAS Number | 1006-41-3 | Molecular Weight | 219.008 | |
| Density | 1.8±0.1 g/cm3 | Boiling Point | 294.3±25.0 °C at 760 mmHg | |
| Molecular Formula | C7H4BrFO2 | Melting Point | 172-176 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 131.8±23.2 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
| Name | 2-Bromo-4-fluorobenzoic acid |
|---|---|
| Synonym | More Synonyms |
| Density | 1.8±0.1 g/cm3 |
|---|---|
| Boiling Point | 294.3±25.0 °C at 760 mmHg |
| Melting Point | 172-176 °C(lit.) |
| Molecular Formula | C7H4BrFO2 |
| Molecular Weight | 219.008 |
| Flash Point | 131.8±23.2 °C |
| Exact Mass | 217.937866 |
| PSA | 37.30000 |
| LogP | 2.31 |
| Vapour Pressure | 0.0±0.7 mmHg at 25°C |
| Index of Refraction | 1.583 |
| InChIKey | RRKPMLZRLKTDQV-UHFFFAOYSA-N |
| SMILES | O=C(O)c1ccc(F)cc1Br |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36-S37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2916399090 |
|
~48% 
2-Bromo-4-fluor... CAS#:1006-41-3 |
| Literature: Mei, Tian-Sheng; Giri, Ramesh; Maugel, Nathan; Yu, Jin-Quan Angewandte Chemie - International Edition, 2008 , vol. 47, # 28 p. 5215 - 5219 |
|
~% 
2-Bromo-4-fluor... CAS#:1006-41-3 |
| Literature: Collection of Czechoslovak Chemical Communications, , vol. 40, # 3 p. 719 - 737 |
|
~% 
2-Bromo-4-fluor... CAS#:1006-41-3 |
| Literature: Journal of Organic Chemistry, , vol. 28, p. 1759 - 1762 |
| Precursor 3 | |
|---|---|
| DownStream 10 | |
| HS Code | 2916399090 |
|---|---|
| Summary | 2916399090 other aromatic monocarboxylic acids, their anhydrides, halides, peroxides, peroxyacids and their derivatives VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:30.0% |
|
Radiosynthesis and in vivo evaluation of 1-[18F]fluoroelacridar as a positron emission tomography tracer for P-glycoprotein and breast cancer resistance protein.
Bioorg. Med. Chem. 19(7) , 2190-8, (2011) Aim of this study was to label the potent dual P-glycoprotein (Pgp) and breast cancer resistance protein (BCRP) inhibitor elacridar (1) with (18)F to provide a positron emission tomography (PET) radio... |
|
|
Regioselective copper-catalyzed amination of bromobenzoic acids using aliphatic and aromatic amines.
J. Org. Chem. 71(8) , 3270-3, (2006) A chemo- and regioselective copper-catalyzed cross-coupling procedure for amination of 2-bromobenzoic acids is described. The method eliminates the need for acid protection and produces N-aryl and N-a... |
|
|
Rapid synthesis and zebrafish evaluation of a phenanthridine-based small molecule library.
Org. Biomol. Chem. 9(7) , 2233-9, (2011) A Heck cyclisation approach is described for the rapid synthesis of a library of natural product-like small molecules, based on the phenanthridine core. The synthesis of a range of substituted benzyla... |
| 4-Fluor-2-brom-benzoesaeure |
| Benzoic acid, 2-bromo-4-fluoro- |
| 2-bromo-4-fluoro-benzoic acid |
| 2-bromo-4-fluorobenzioc acid |
| 4-fluoro-2-bromo-benzoic acid |
| RARECHEM AL BO 1996 |
| 3-Bromo-4-fluorobenzoic acid |
| 2-Bromo-4-Fluorobenzoic |
| 2-Brom-4-fluor-benzoesaeure |
| 2-Bromo-4-fluorobenzoicacid |
| MFCD00055370 |
| 2-Bromo-4-fluorobenzoic acid |
| 2-Bromofluorobenzoic Acid |
| Benzoic acid, 3-bromo-4-fluoro- |


 CAS#:36282-26-5
CAS#:36282-26-5 CAS#:446-32-2
CAS#:446-32-2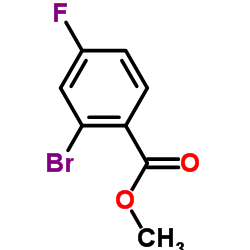 CAS#:653-92-9
CAS#:653-92-9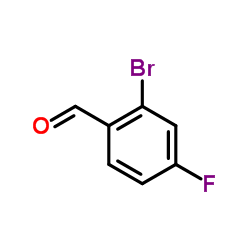 CAS#:59142-68-6
CAS#:59142-68-6 CAS#:1006-40-2
CAS#:1006-40-2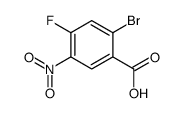 CAS#:1036389-83-9
CAS#:1036389-83-9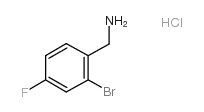 CAS#:289038-14-8
CAS#:289038-14-8 CAS#:229027-89-8
CAS#:229027-89-8 CAS#:1203662-37-6
CAS#:1203662-37-6
