Desloratadine
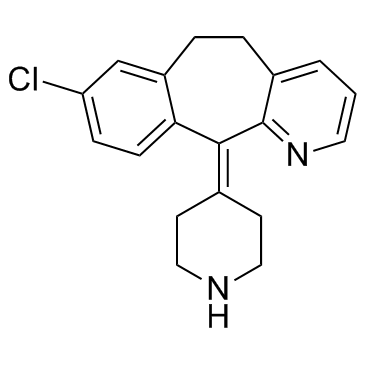
Desloratadine structure
|
Common Name | Desloratadine | ||
|---|---|---|---|---|
| CAS Number | 100643-71-8 | Molecular Weight | 310.821 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 467.9±45.0 °C at 760 mmHg | |
| Molecular Formula | C19H19ClN2 | Melting Point | 150-151°C | |
| MSDS | Chinese USA | Flash Point | 236.8±28.7 °C | |
Use of DesloratadineDesloratadine(Sch34117) is a potent antagonist for human histamine H1 receptor used to treat allergies.Target: Histamine H1 ReceptorDesloratadine binds to the human H1 receptor with Ki value of 0.87 nM in displacing tritiated mepyramine. Desloratadine (100 nM to 10 μM) inhibits both IgE-mediated and non-IgE-mediated generation of the cytokines IL-4 and IL-13 by human basophils. Desloratadine (300 nM to 100 μM) inhibits both IgE and non-IgE-mediated histamine release from human peripheral blood basophils. Desloratadine (0.1 μM to 10 μM) is also shown to inhibit platelet-activating factor-induced eosinophil chemotaxis and TNF-α-induced eosinophil adhesion in eosinophils obtained from patients with allergic rhinitis or allergic asthma [1]. Desloratadine (1 μM-10 μM) dose-dependently inhibits the release of histamine and LTC4 from human basophils. Desloratadine (0.1 μM-10 μM) dose-dependently inhibits IL-13 secretion from basophils activated with IL-3 and PMA from human basophils. Desloratadine (10 μM) pretreatment results in a substantial decrease of the induced cytokine message in cultured basophils. Desloratadine (10 μM) pretreatment causes approximately an 80% reduction in the IL-4 message accumulated with anti-IgE activation in cultured basophils. Desloratadine (10 μM) also inhibits the histamine and IL-4 protein secreted into the supernatants of cultured basophils [2]. [3H]Desloratadine binds to the human histamine H1 receptor expressed in CHO cells with Kd of 1.1 nM. Desloratadine is 52, 57, 194, and 153 times more potent than cetirizine, ebastine, fexofenadine, and loratadine, respectively, in competition-binding studies [3]. |
| Name | desloratadine |
|---|---|
| Synonym | More Synonyms |
| Description | Desloratadine(Sch34117) is a potent antagonist for human histamine H1 receptor used to treat allergies.Target: Histamine H1 ReceptorDesloratadine binds to the human H1 receptor with Ki value of 0.87 nM in displacing tritiated mepyramine. Desloratadine (100 nM to 10 μM) inhibits both IgE-mediated and non-IgE-mediated generation of the cytokines IL-4 and IL-13 by human basophils. Desloratadine (300 nM to 100 μM) inhibits both IgE and non-IgE-mediated histamine release from human peripheral blood basophils. Desloratadine (0.1 μM to 10 μM) is also shown to inhibit platelet-activating factor-induced eosinophil chemotaxis and TNF-α-induced eosinophil adhesion in eosinophils obtained from patients with allergic rhinitis or allergic asthma [1]. Desloratadine (1 μM-10 μM) dose-dependently inhibits the release of histamine and LTC4 from human basophils. Desloratadine (0.1 μM-10 μM) dose-dependently inhibits IL-13 secretion from basophils activated with IL-3 and PMA from human basophils. Desloratadine (10 μM) pretreatment results in a substantial decrease of the induced cytokine message in cultured basophils. Desloratadine (10 μM) pretreatment causes approximately an 80% reduction in the IL-4 message accumulated with anti-IgE activation in cultured basophils. Desloratadine (10 μM) also inhibits the histamine and IL-4 protein secreted into the supernatants of cultured basophils [2]. [3H]Desloratadine binds to the human histamine H1 receptor expressed in CHO cells with Kd of 1.1 nM. Desloratadine is 52, 57, 194, and 153 times more potent than cetirizine, ebastine, fexofenadine, and loratadine, respectively, in competition-binding studies [3]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 467.9±45.0 °C at 760 mmHg |
| Melting Point | 150-151°C |
| Molecular Formula | C19H19ClN2 |
| Molecular Weight | 310.821 |
| Flash Point | 236.8±28.7 °C |
| Exact Mass | 310.123688 |
| PSA | 24.92000 |
| LogP | 6.77 |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.626 |
| Storage condition | 2-8°C |
| Hazard Codes | Xi |
|---|---|
| Risk Phrases | R11:Highly Flammable. |
| Safety Phrases | S16-S23-S36/37/39 |
| RIDADR | 1993 |
| Precursor 9 | |
|---|---|
| DownStream 2 | |
|
Utility of cerebrospinal fluid drug concentration as a surrogate for unbound brain concentration in nonhuman primates.
Drug Metab. Pharmacokinet. 29(5) , 419-26, (2014) In central nervous system drug discovery, cerebrospinal fluid (CSF) drug concentration (C(CSF)) has been widely used as a surrogate for unbound brain concentrations (C(u,brain)). However, previous rod... |
|
|
A long-standing mystery solved: the formation of 3-hydroxydesloratadine is catalyzed by CYP2C8 but prior glucuronidation of desloratadine by UDP-glucuronosyltransferase 2B10 is an obligatory requirement.
Drug Metab. Dispos. 43(4) , 523-33, (2015) Desloratadine (Clarinex), the major active metabolite of loratadine (Claritin), is a nonsedating long-lasting antihistamine that is widely used for the treatment of allergic rhinitis and chronic idiop... |
|
|
Differential thermodynamic driving force of first- and second-generation antihistamines to determine their binding affinity for human H1 receptors.
Biochem. Pharmacol. 91(2) , 231-41, (2014) Differential binding sites for first- and second-generation antihistamines were indicated on the basis of the crystal structure of human histamine H1 receptors. In this study, we evaluated differences... |
| 8-Chlor-11-piperidin-4-yliden-6,11-dihydro-5H-benzo[5,6]cyclohepta[1,2-b]pyridin |
| 8-Chloro-6,11-dihydro-11-(4-piperidinylidene)-5H-benzo[5,6]cyclohepta[1,2-b]pyridine |
| UNII-FVF865388R |
| AzoMyr |
| 5H-Benzo[5,6]cyclohepta[1,2-b]pyridine, 8-chloro-6,11-dihydro-11-(4-piperidinylidene)- |
| DESLORATIDINE |
| MFCD00871949 |
| Opulis |
| Desloratadine |
| 8-Chloro-11-(4-piperidinylidene)-6,11-dihydro-5H-benzo[5,6]cyclohepta[1,2-b]pyridine |
| Allex |
| Descarboethoxyoratidine |
| Neoclarityn |
| Aerius |
| Sch34117 |
| descarboethoxyloratadine |
| 8-chloro-11-(piperidin-4-ylidene)-6,11-dihydro-5H-benzo[5,6]cyclohepta[1,2-b]pyridine |
| Clarinex |
 CAS#:79794-75-5
CAS#:79794-75-5 CAS#:38092-89-6
CAS#:38092-89-6 CAS#:32998-95-1
CAS#:32998-95-1 CAS#:31255-57-9
CAS#:31255-57-9![3-[2-(3-CHLORO-PHENYL)-ETHYL]-PYRIDINE-2-CARBOXYLIC ACID TERT-BUTYLAMIDE Structure](https://image.chemsrc.com/caspic/097/107285-30-3.png) CAS#:107285-30-3
CAS#:107285-30-3![(1-Methyl-4-piperidinyl) {3[2-(3-chlorophenyl) ethyl ]2-pyridinyl} methanone Structure](https://image.chemsrc.com/caspic/382/130642-50-1.png) CAS#:130642-50-1
CAS#:130642-50-1 CAS#:620-20-2
CAS#:620-20-2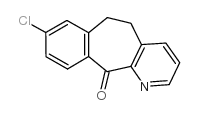 CAS#:31251-41-9
CAS#:31251-41-9 CAS#:63463-36-5
CAS#:63463-36-5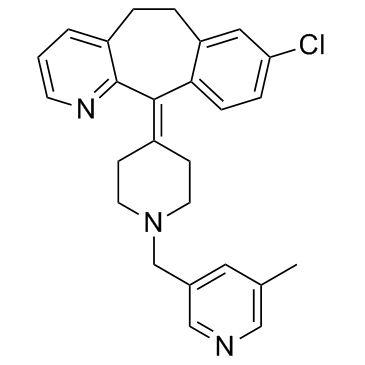 CAS#:158876-82-5
CAS#:158876-82-5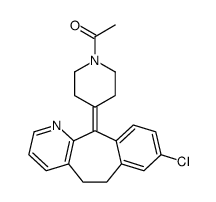 CAS#:117796-52-8
CAS#:117796-52-8
