Methyl indole-5-carboxylate

Methyl indole-5-carboxylate structure
|
Common Name | Methyl indole-5-carboxylate | ||
|---|---|---|---|---|
| CAS Number | 1011-65-0 | Molecular Weight | 175.184 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 331.7±15.0 °C at 760 mmHg | |
| Molecular Formula | C10H9NO2 | Melting Point | 126-128 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 154.4±20.4 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
| Name | Methyl indole-5-carboxylate |
|---|---|
| Synonym | More Synonyms |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 331.7±15.0 °C at 760 mmHg |
| Melting Point | 126-128 °C(lit.) |
| Molecular Formula | C10H9NO2 |
| Molecular Weight | 175.184 |
| Flash Point | 154.4±20.4 °C |
| Exact Mass | 175.063324 |
| PSA | 42.09000 |
| LogP | 2.58 |
| Vapour Pressure | 0.0±0.7 mmHg at 25°C |
| Index of Refraction | 1.639 |
| InChIKey | DRYBMFJLYYEOBZ-UHFFFAOYSA-N |
| SMILES | COC(=O)c1ccc2[nH]ccc2c1 |
| Storage condition | Refrigerator |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2933990090 |
| Precursor 7 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Synthesis and antimalarial testing of neocryptolepine analogues: addition of ester function in SAR study of 2,11-disubstituted indolo[2,3-b]quinolines.
Eur. J. Med. Chem. 64 , 498-511, (2013) This report describes the synthesis, and in vitro and in vivo antimalarial evaluations of certain ester-modified neocryptolepine (5-methyl-5H-indolo[2,3-b]quinoline) derivatives. The modifications wer... |
|
|
Anticancer activity of MPT0E028, a novel potent histone deacetylase inhibitor, in human colorectal cancer HCT116 cells in vitro and in vivo.
PLoS ONE 7(8) , e43645, (2012) Recently, histone deacetylase (HDAC) inhibitors have emerged as a promising class of drugs for treatment of cancers, especially subcutaneous T-cell lymphoma. In this study, we demonstrated that MPT0E0... |
|
|
Copper-catalyzed cross dehydrogenative coupling reactions of tertiary amines with ketones or indoles.
Org. Lett. 12 , 5214, (2010) A novel cross dehydrogenative coupling (CDC) reaction of N,N-dimethylanilines with methyl ketones by cooperative copper and aminocatalysis has been developed, which leads to the formation of β-arylami... |
| Indole-5-carboxylic acid,methyl ester |
| Methyl 1H-indole-5-carboxylate |
| 1H-Indole-5-carboxylic acid, methyl ester |
| MFCD00153023 |
| Indole-5-carboxylic Acid Methyl Ester |
| Methyl indole-5-carboxylate |
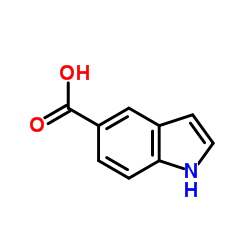 CAS#:1670-81-1
CAS#:1670-81-1 CAS#:77-78-1
CAS#:77-78-1 CAS#:74-88-4
CAS#:74-88-4 CAS#:1670-82-2
CAS#:1670-82-2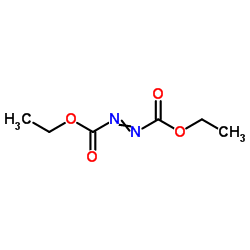 CAS#:1972-28-7
CAS#:1972-28-7 CAS#:54509-73-8
CAS#:54509-73-8 CAS#:126759-30-6
CAS#:126759-30-6![methyl 1-[(3-nitrophenyl)methyl]indole-5-carboxylate structure](https://image.chemsrc.com/caspic/036/141451-73-2.png) CAS#:141451-73-2
CAS#:141451-73-2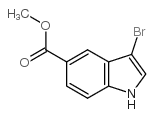 CAS#:916179-88-9
CAS#:916179-88-9 CAS#:197506-83-5
CAS#:197506-83-5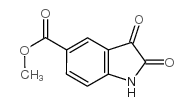 CAS#:101460-85-9
CAS#:101460-85-9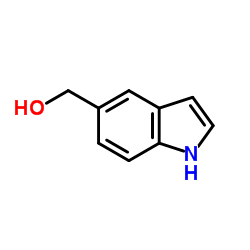 CAS#:1075-25-8
CAS#:1075-25-8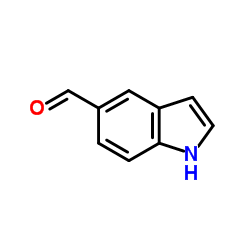 CAS#:1196-69-6
CAS#:1196-69-6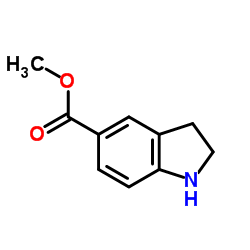 CAS#:141452-01-9
CAS#:141452-01-9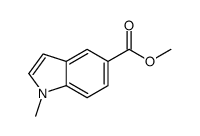 CAS#:128742-76-7
CAS#:128742-76-7 CAS#:15861-30-0
CAS#:15861-30-0
