Ac-D-Phe-OH
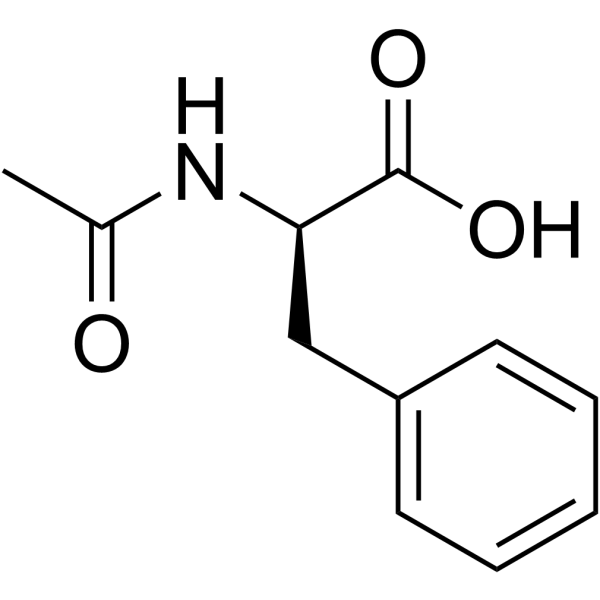
Ac-D-Phe-OH structure
|
Common Name | Ac-D-Phe-OH | ||
|---|---|---|---|---|
| CAS Number | 10172-89-1 | Molecular Weight | 207.226 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 453.9±38.0 °C at 760 mmHg | |
| Molecular Formula | C11H13NO3 | Melting Point | 167ºC | |
| MSDS | Chinese USA | Flash Point | 228.3±26.8 °C | |
Use of Ac-D-Phe-OHN-Acetyl-D-phenylalanine is a phenylalanine derivative[1]. |
| Name | N-acetyl-D-phenylalanine |
|---|---|
| Synonym | More Synonyms |
| Description | N-Acetyl-D-phenylalanine is a phenylalanine derivative[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Amino acids and amino acid derivatives have been commercially used as ergogenic supplements. They influence the secretion of anabolic hormones, supply of fuel during exercise, mental performance during stress related tasks and prevent exercise induced muscle damage. They are recognized to be beneficial as ergogenic dietary substances[1]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 453.9±38.0 °C at 760 mmHg |
| Melting Point | 167ºC |
| Molecular Formula | C11H13NO3 |
| Molecular Weight | 207.226 |
| Flash Point | 228.3±26.8 °C |
| Exact Mass | 207.089539 |
| PSA | 66.40000 |
| LogP | 0.78 |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.548 |
| Storage condition | −20°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2924299090 |
| Precursor 8 | |
|---|---|
| DownStream 2 | |
| HS Code | 2924299090 |
|---|---|
| Summary | 2924299090. other cyclic amides (including cyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Peptide synthesis catalyzed by an antibody containing a binding site for variable amino acids.
Science 265 , 234, (1994) Monoclonal antibodies, induced with a phosphonate diester hapten, catalyzed the coupling of p-nitrophenyl esters of N-acetyl valine, leucine, and phenylalanine with tryptophan amide to form the corres... |
|
|
Studies on the mechanism for renal elimination of N-acetylphenylalanine: its pathophysiologic significance in phenylketonuria.
J. Lab. Clin. Med. 105(1) , 132-8, (1985) To elucidate the mechanism and biologic significance of urinary occurrence of N-acetylphenylalanine in phenylketonuria, the metabolic fate of N-acetylphenylalanine was studied in rats. In vivo and in ... |
|
|
Molecular dynamics (MD) simulations for the prediction of chiral discrimination of N-acetylphenylalanine enantiomers by cyclomaltoheptaose (beta-cyclodextrin, beta-CD) based on the MM-PBSA (molecular mechanics-Poisson-Boltzmann surface area) approach.
Carbohydr. Res. 339(11) , 1961-6, (2004) Molecular dynamics (MD) simulations were performed for the prediction of chiral discrimination of N-acetylphenylalanine enantiomers by cyclomaltoheptaose (beta-cyclodextrin, beta-CD). Binding free ene... |
| D-Phenylalanine, N-acetyl- |
| N-Acetyl-D-phenylalanine |
| MFCD00002664 |
| Acetyl-D-Phenylalanine |
| (2R)-2-acetamido-3-phenylpropanoic acid |
| Ac-D-Phe-OH |
| EINECS 233-447-7 |
 CAS#:673-06-3
CAS#:673-06-3 CAS#:108-24-7
CAS#:108-24-7 CAS#:5429-56-1
CAS#:5429-56-1 CAS#:4134-09-2
CAS#:4134-09-2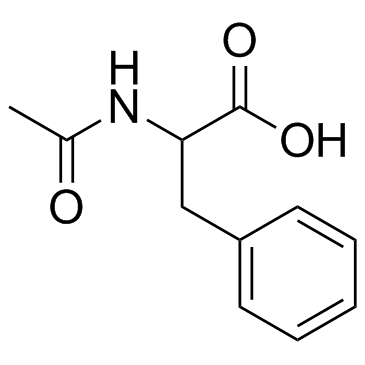 CAS#:2901-75-9
CAS#:2901-75-9![(E)-N-[(2-furylmethylideneamino)carbamoylmethyl]-3-phenyl-prop-2-enamide Structure](https://image.chemsrc.com/caspic/297/5469-44-3.png) CAS#:5469-44-3
CAS#:5469-44-3 CAS#:100350-85-4
CAS#:100350-85-4 CAS#:21156-62-7
CAS#:21156-62-7
